Ethics, COVID-19 & Africa
Publishing During the COVID-19 Epidemic: Prompt Conclusions or Quality Studies?
FOR COMPREHENSIVE COVID-19 ETHICS AND POLICY RESOURCES, VISIT OUR DEDICATED WEBPAGE, BIOETHICS.JHU.EDU/CORONAVIRUS.
By Michael Erdek, MD, MA
Associate Professor
Johns Hopkins Berman Institute of Bioethics and School of Medicine
The current COVID-19 health crisis warrants dissemination of outcomes from clinical trials in a relatively prompt fashion given the steep slope of the infection curve and the concomitant dire predictions regarding both patient care and resource utilization. It thus stands to reason that researchers are motivated to turn around data rapidly, consistent with serving the common good in time of extreme need. This process, however, has its limits when underpowered studies with limited data yield conclusions with broad-reaching implications.
There is little doubt that an urgent need for treatment of SARS-CoV-2 exists. But premature publication of definitive recommendations based on inappropriate conclusions grounded in scant, hastily-acquired data serve only at best to confuse and at worst mislead at a time when tensions are high and need for help is great. One recent study by Gautret etal., “Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open label non-randomized clinical trial,” published in the International Journal of Antimicrobial Agents and receiving attention all the way to the White House, raises such concerns.
In this investigation, 36 SARS-Co-V-2 positive adult patients were studied over a two-week period at a hospital in Marseille, France. Twenty patients received 200 mg of hydroxychloroquine sulfate three times per day for 10 days while 16 patients served as control subjects. Six of those randomized to the active treatment also received 500 mg of azithromycin on Day 1 and then subsequently 250 mg per day for four additional days at the authors’ discretion “depending on clinical presentation” in order “to prevent bacterial superinfection.”
The results detail that at Day 6, 100% of those treated with combination hydroxychloroquine and azithromycin demonstrated virological cure (per negative nasopharyngeal PCR results), compared with 57.1% of those receiving hydroxychloroquine only and 12.5% in the control group (p<0.001). The recommendation issued by the authors was thus that “COVID-19 patients be treated with hydroxychloroquine and azithromycin.”
Publication of recommendations based on studies such as this is highly problematic. An investigation with only 20 treated patients (after six dropped out due to cessation of therapy) is significantly underpowered and greatly subject to sample size error. Further, the reason for the addition of azithromycin is poorly described and subject to question. It is one thing to call results promising, it is another to issue definitive treatment recommendations. Hydroxychloroquine exists in limited supply, and poorly grounded recommendations touting effectiveness by health care providers and/or President Trump who touted it last week as “a game-changer” that has shown “very, very encouraging results,” only exacerbate this shortfall without correlative evidence of demonstrable benefit. This is even more troubling with regard to azithromycin, where overuse may lead to resultant potential shortages of an important antibiotic needed to treat bacterial infection as well as the downstream effect of increasing the number of strains of drug-resistant pathogens.
It is ironic that the authors of this study state, “For ethical reasons and because our first results are so significant and evident we decide to share our findings with the medical community, given the urgent need for an effective drug against SARS-CoV-2 in the current pandemic context.” The true ethical responsibility of researchers is to produce and to publish data and subsequent recommendations yielded as a function of robust methodology and stringent review. Perhaps some investigators may be altruistically motivated in an effort to rapidly disseminate hopeful conclusions. Nevertheless, the responsibility we hold to our patients and, especially in the setting of the current pandemic, to greater society at large depends on good judgment and fiduciary concern rather than on haste to demonstrate beneficial outcomes.
Research Ethics Consultation Service
Join the Berman Institute at ASBH 2019

The Berman Institute will be well represented at the 21st annual meeting of the American Society for Bioethics and Humanities (ASBH), as a group of faculty, fellows, and students are headed to Pittsburgh, PA, October 24 – 27, 2019.
Preview our diverse presentations (full program available online), and plan a visit to our booth in the ASBH exhibit hall replete with BI swag – (including this year’s limited edition wood cube basil planter!)
 |
You can also follow us on Twitter: #ASBH19, featuring our @bermaninstitute, @aregenberg, @kahnethx, @tnrethx, @DiStefano_MJ, and more. |
|
SPECIAL EVENTS:Thursday, Oct. 24, 7:30 pm-9:30pm: Book Release Party – Celebrating recent Oxford University Press Books published by Berman Institute Faculty. Friday, Oct. 25, 6:00 pm-8:00 pm: Reception – hosted by the Johns Hopkins Berman Institute and the Wellcome Trust.
|
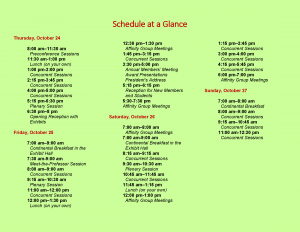
Full Schedule:
–Thursday, October 24–
Concurrent Sessions – 1:00 pm–2:00 pm | Location: Room 303
Improving Informed Consent in Research
Joseph Ali, JD; Holly Taylor, PhD; Nae-Yuh Wang, PhD; Daphne Washington, BA

Informed consent documents remain too long, complex and legalistic, threatening the ability of even committed potential research participants to understand research. Recent changes to U.S. regulations (Common Rule), as well as significant bioethics literature, suggest consent forms should be shorter and simpler. Despite decades of consent research, there is no simple, adaptable, evidence-based approach to improving participant understanding of research. Robust study of consent interventions in diverse, non-simulated research settings is critically important to effective communication of research information and adaptation of new (presently vague) consent “key information” requirements. In this session, we present the methods and core findings from a Greenwall Foundation-funded three-arm randomized consent intervention study conducted at a large research-intensive institution. This study developed and tested two reproducible consent approaches that are concise, emphasize key information, provide information in discrete chunks, and use lay language. Individuals considering enrollment across five ongoing clinical studies (target n = 300 participants) were randomized to receive either 1) the standard consent form and process (control), 2) a briefer consent fact sheet, or 3) a video consent summary based on the fact sheet and consisting of a conversation between the study PI and an actor playing a potential participant. A previously developed tool (Consent Understanding Evaluation) was modified to measure both consent understanding and satisfaction. Data collection will be complete by April 2019. Analysis will be complete by June, with methods and key findings ready to share for October.
1:00 pm-2:00 pm | Location: Room 304-305
The Ethics of Harm Research with Nonhuman Animals
Anne Barnhill, PhD, Jake Earl, PhD, David Wendler, PhD

Over the past several decades, conversation and collaboration among bioethicists, scientists, and policy makers have led to substantial improvements in the well-being of nonhuman animal subjects in biomedical research. While this progress is commendable, there are no regulations that limit the nature or amount of harm that researchers may impose on nonhuman animal subjects. Should there be any limits on the sorts of harm that nonhuman animal subjects suffer, and if so, what should those limits be? Each of the three panelists offers a distinctive perspective on the justifiability of imposing serious harms on nonhuman animal subjects, which together have significant ethical and policy implications for the conduct of biomedical research. The first panelist will set the stage by surveying the ethical issues raised by infection challenge trials with nonhuman primates. These studies are an excellent test case in that they impose serious harms on cognitively sophisticated animals while providing invaluable information for fighting disease in humans. The second panelist will argue that there are no harms that in principle cannot permissibly be imposed on most nonhuman animals, including most nonhuman primates. This is because unlike most humans, most nonhuman animals can be fully compensated for any harms they might suffer in research. The third panelist will argue that there are some harmful experiences that are so intrinsically bad that they cannot be fully compensated, and therefore the permissibility of harming nonhuman animal subjects in biomedical research will depend on answers to difficult questions about the nature and value of suffering.
1:00 pm – 2:00 pm | Location: Room 321
Disparities in Access to Fertility Care in the United States: Ethical Considerations for Equitable Solutions

There are significant disparities in access to fertility services in the United States. These disparities exist along lines of race, ethnicity, education, income, geographic location, marital status, gender identity, sexual orientation and comorbid conditions (e.g., HIV, Hepatitis C). In this paper, I will argue that these inequalities represent injustices and therefore demand rectification. The disparities in access to care exist for certain groups with shared characteristics and these groups are overall socially and historically disadvantaged.
However, proposed solutions will also raise ethical issues that require careful consideration. For example, passing a policy that mandates insurance coverage of in vitro fertilization raises questions about eligibility, resource allocation, distributive justice and limitations on which reproductive technologies ought to be covered. Such a policy also fails to address barriers other than cost, such as geographic location, which may perpetuate existing disparities. Given that barriers to care include financial, geographical, and sociocultural factors, I will argue for the necessity of multifaceted solutions. Ultimately, my investigation reveals that proposed solutions to reduce disparities in access to fertility care raise ethical issues of their own. These issues are not reasons for inaction, but rather ought to be carefully considered when evaluating policy solutions. I will conclude with recommendations for mitigating ethical concerns that arise from policies that aim to address disparities in access to fertility care in the United States.
2:15 pm-3:45 pm | Location: Room 317-318
The Hastings Center 2019 Henry Beecher Award Session

Ruth Faden, PhD, MPH, Philip Franklin Wagley Professor of Bioethics, Johns Hopkins University is the 2019 recipient of The Beecher Award for Lifetime Achievement, which is The Hastings Center’s most prestigious award for life time achievement. Founder of the Johns Hopkins Berman Institute of Bioethics, Dr. Faden’s work has profoundly influenced innumerable aspects of our field and, perhaps more importantly, helped to shape public policy on some of the nation’s most pressing issues: from HIV testing of pregnant women to food and agriculture policy, and many aspects of science policy, including stem cell and embryo research. She has also powerfully critiqued the prevailing research ethics paradigm in the United States, encouraging major shifts in how we think about the oversight of comparative effectiveness research. In 1994-5, she chaired the United States Advisory Committee on Human Radiation Experiments, which issued a report President Bill Clinton said “should be engraved in our national memory.” In her latest book, Structural Injustice: Power, Advantage, and Human Rights (September 2019, Oxford University Press), Dr. Faden and co-author Madison Powers build on their longstanding call for bioethics to expand its understanding of justice to put forward a groundbreaking theory of social injustice, more broadly. Their theory forges links between human rights and fairness norms and is built to fit a real-world characterized by deprivation, human rights violations, disadvantage, and unfair power relations, both within and across nations. This session will also include presentation of the inaugural David Roscoe Early Career Essay Award. Through this award, which includes a $2,000 prize, The Hastings Center aims to identify and celebrate early-career post-docs and junior faculty for excellence in a published paper that has brought fresh analysis to the social and ethical implications of advances in science and technology. This year’s award winner will be named for the first time at this event. The session will include introductory comments by Mildred Solomon, president of The Hastings Center, who will then interview Professor Faden, in a wide-ranging discussion of her reflections on her career, the major issues she has addressed, and where she would like to see the field going in the future. David Roscoe, the former long-time chair of The Hastings Center, and now chair of its Advisory Committee, will present the Roscoe Essay Award and its recipient will make brief remarks. There will be plenty of time for questions from the audience.
2:15 pm-3:45 pm | Location: Room 310-311
Integrating Procedural and Substantive Values to Assess the Ethics of Process in the Healthcare Priority Setting

High quality universal health coverage is an increasingly important policy objective for many countries, especially low- and middle-income countries. While health care demand may be infinite, resources for health are finite. Health care priority-setting is thus inescapable, leaving countries with difficult choices regarding what services will be covered by publicly funded health systems. In discussions of the ethics of health care priority-setting, two questions dominate: 1) what substantive criteria ought to inform priority-setting decisions, and 2) what processes ought to guide priority-setting? These questions are typically explored independently. For instance, Accountability for Reasonableness – the dominant account of process – was developed to achieve fair and legitimate priority-setting decisions independent of the substantive criteria chosen to inform those decisions. I defend a novel approach for assessing the ethics of process in health care priority-setting that integrates procedural and substantive ethics considerations to address key critiques of and expand on Accountability for Reasonableness. I show how this novel approach provides better guidance for adapting to specific national contexts and gives much needed attention to values beyond fairness and legitimacy. I also discuss novel benefits of pursuing parallel and mutually coherent – rather than independent – substantive and procedural ethics frameworks for health care priority-setting. First, substantive values beyond fairness such as respect and solidarity may provide additional or stronger justification for the selection and design of process elements. Second, process elements may help decision-makers properly apply a substantive value – such as equity – when assessing an intervention for coverage.
4:00 pm-5:00 pm | Location: Room 302
A Drama of Resilience and Remembrance: Reflecting on Life, Loss, and Liminality
Lynn Bush PhD, MS, MA, Bob Truog MD, MA, Christine Mitchell RN, MS, MTS, FAAN, Cheryl Lew, MD, MSEd, MSBioethics, FAAP, Karen Rothenberg, JD, MPA, Ben Wilfond, MD

In remembrance of the Tree of Life tragedy our host city experienced and the extraordinary resilience demonstrated in the aftermath of such trauma and loss, a new dramatic vignette-play provides a stage for the ASBH community to explore the conference’s thematic concepts in the symbolic context of a vastly different “tree of life” scenario. Set in a pediatric hospital, the drama spotlights life, loss, and liminality surrounding some ethically controversial cutting-edge technologies for which Pittsburgh and the geographically diverse panelists’/actors’ medical centers are among the field’s leaders, including regenerative and gene therapies for rare diseases as well as “elective” organ transplantation to improve quality of life and experimental fetal therapies. Utilizing dramatic narrative-vignette pedagogy, the twenty minute play stimulates interprofessional dialogue among ASBH bioethics and humanities-scholars about ethical dilemmas and psychosocial challenges raised by the characters. The session will critically examine complex issues facing our multicultural society as we implement these technologies and address concepts of normalcy and illness; decision-making evoking hope and grief; along with concerns about health disparities, justice, and equity in access to limited resources, including consideration for the disability community. Following the play, panelists (PhD; RN; MDs; all experienced in creative educational approaches) will present their varying perspectives, and actively engage the actors and audience in 40 minutes of robust discussion reflecting on the ethical justifications and policy implications of therapeutic boundaries when the individuals are vulnerable, the resources are limited, the disorders are rare, the evidence is not clear, and the treatments are not trivial. [Actor-panelists include: Lynn Bush, Bob Truog, Christine Mitchell, Cheryl Lew, Karen Rothenberg, Ben Wilfond].
4:00 pm-5:00 pm | Location: Room 316


4:00 pm-5:00 pm | Location: Room 303

7:30 pm-9:30 pm | Location: The Westin Pittsburgh, Butler Room
Book Release Party
The Johns Hopkins Berman Institute of Bioethics invites you to a Book Release Party (dinner will be served) celebrating recent Oxford University Press books by Berman Institute Faculty:
Beyond Consent (2nd Edition)
Jeffrey P. Kahn, Anna C. Mastroianni, and Jeremy Sugarman, eds.
Structural Injustice: Power, Advantage, and Human Rights
Madison Powers and Ruth Faden
The Oxford Handbook of Public Health Ethics
Anna C. Mastroianni, Jeffrey P. Kahn, and Nancy Kass, eds.
–Friday, October 25–
Concurrent Sessions – 8:00 – 9:00 am | Location: Room 306-307
“Because It’s My Child and I Want to Know”: A Survey of Pregnant Women’s Views on the Utility of Ultrasound Screening for Fetal Anomalies
Margot Kelly-Hedrick, MBE, Marielle S. Gross, MD, MBE


Screening ultrasounds are routinely performed during pregnancy, primarily to diagnose major fetal anomalies that may influence women’s decision to have an abortion. As ultrasounds have grown more sophisticated, women are also receiving more detailed findings with variable clinical significance. Meanwhile, some women undergoing screening ultrasounds may not consider abortion regardless of findings, whereas others may find their access to abortion increasingly limited. Little is known about how women view the utility of prenatal anomaly screens.
We surveyed 286 pregnant women immediately before scheduled ultrasounds at an academic tertiary care center. Quantitative and qualitative questions explored views surrounding ultrasound results and abortion for fetal anomalies. Almost all women wanted to know if there were abnormal ultrasound findings; thematic analysis of reasons included being prepared, valuing awareness, and the importance of results for clinical decision-making. Women reported both general (e.g., baby’s health) and specific worries (e.g., Down syndrome) about the upcoming test. About half would consider abortion for anomalies; most focused on fetal prognosis and a minority discussed maternal considerations. Those who would not consider an abortion were most likely to cite personal or religious reasons. In this population, white women were significantly more likely than black women to consider an abortion for fetal anomalies.
These findings highlight women’s perceptions of prenatal ultrasounds not only as informing clinical decision-making – either for abortion or interventions for fetal benefit – but also as an inherently valuable source of information and preparedness. In closing, we discuss implications these findings may have for clinical practice.
8:00 – 9:00 am | Location: Room 315
Ethical Challenges Facing Healthcare Workers in Settings of Extreme Violence
Matthew DeCamp, MD PhD; Grant Broussard, MSPH; Courtland Robinson, PhD; Leonard S. Rubenstein, JD

- Despite their protection under international law, attacks against health care facilities and workers occur with alarming frequency. During the war in Syria alone, more than 500 attacks have occurred against hospitals. The effects of these attacks devastatingly deprive access to health care for local populations, harm or kill health care workers, and disrupt core social institutions. Individuals and aid organizations working in these settings of extreme violence experience profoundly difficult choices, ranging from how to maintain an acceptable standard of care amidst chaos to whether to rebuild facilities in the same place (at risk of repeat attacks) or in a remote one (at risk of reducing accessibility to people in need). Here, we report findings from a multi-year project examining ethical challenges faced by health care workers and aid organizations operating in Syria. Through a systematic literature review, semi-structured interviews, and in-country workshops, our project described the full range of ethical challenges experienced in settings of extreme violence. These challenges were categorized according to eight core ethical obligations. Our analysis found areas of overlap, tension, and mutual reinforcement between the humanitarian principles to which humanitarian organizations are committed (i.e., neutrality, impartiality, humanity, and independence) and these ethical obligations. Our research suggests that, by incorporating ethical principles and frameworks for ethical decision-making, organizations operating in settings of extreme violence can find novel ways of fulfilling core humanitarian principles and managing the moral distress inherent in these settings. Five recommendations are proposed for how humanitarian organizations could operationalize ethical decision-making.
11:00 am – 12:00 pm | Location: Room 304-305
Global Health Justice and Governance
Anne Barnhill, PhD, Matthew McCoy, PhD, MA, MS, Jennifer Prah Ruger, PhD, MSc, MA, MSL, Eric Juengst, PhD, Danielle Wenner, PhD, Alex J. London, PhD

This panel will bring together four panelists around the common theme of global heath justice and governance comparing and contrasting perspectives from law and governance, political theory and public policy, global public health ethics and policy, and global health justice and governance, commenting on the recently published book from Oxford University Press on this topic. In a world beset by serious health disparities, by dangerous contagions that can circle the globe in hours, and by a confusion of health actors and systems, humankind needs a new vision, a new architecture, new coordination among renewed systems to ensure health for all. Global Health Justice and Governance lays out the critical problems facing the world today and offers a theory of justice and governance as a way to resolve these seemingly intractable issues. Panelists in this session will offer various perspectives on issues around this common theme of global health justice and governance, such as domestic and international health law and public health law, public preferences and debate about health, ethical issues related to resource allocation and policy, ethics of global public health and disease prevention in diverse societies, political theory and public and private institutions and organizations.
6:00 pm-8:00 pm | Location: Union Standard, 524 William Penn Place, Pittsburgh, PA
The Johns Hopkins Berman Institute and
the Wellcome Trust Reception
The Johns Hopkins Berman Institute of Bioethics and the Wellcome Trust invite you to a reception at the 2019 American Society for Bioethics + Humanities Meeting
–Saturday, October 26–
Concurrent Sessions – 10:45 – 11:45 am | Location: Room 309
Distrust Due to Injustice: How Should Health Pactitioners Respond?
Justin Bernstein, PhD, Joseph Ali, JD


Bi-directional trust is essential to the effective practice of medicine, research and public health globally. What influences individual and collective understanding and ability to maintain trust is interesting in its own right. In this panel, we wish to consider the moral and political significance of distrust in cases where patients and populations have been impacted by historical or ongoing abuses of trust. We will focus on two concrete cases to illustrate the nature of “distrust due to injustice” in public health and implications for response. The first case will illustrate the effect of distrust due to injustice on the collection and storage of health-related information to support development of population estimates of burdens of disease in low-resource countries. The second will reflect on vaccine hesitancy amongst some African Americans due to historical and ongoing betrayals of trust in the medical context. In such cases, we wish to consider not only what sorts of efforts ought to be made to mitigate the relevant form of distrust, but also what sorts of obligations of justice arise in light of this distrust, longer-term steps that ought to be undertaken to fulfill these obligations, as well as how these obligations should shape the relationship between patient and health worker, citizen, and state. We intend to incorporate perspectives from ethics, political philosophy, and public health in engaging with these questions.
1:15 pm-2:45 pm | Location: Room 306-307
Bioethics Book Club: Treating Pain During North America’s Opioid Epidemic
Travis N. Rieder, PhD, Anita Ho, PhD, MPH, Daniel Z. Buchman, PhD, MSW


The goal of this panel is to bring a “book club” feel to ASBH, encouraging broad participation while ensuring rigorous scholarly commentary. The subject of the panel will be a new book (out summer 2019) on the ethics of opioid use in pain management against the backdrop of North America’s opioid crisis. The central question of the book is: what does responsible use of this medication look like, given its role in today’s opioid overdose crisis? Intended to reach a broader audience, the text is written by a bioethics scholar to bring crucial bioethics reasoning to the general public. The author (Panelist 1) will open the discussion with a 15-minute précis before turning it over to the other main discussants. They will then provide 15-minute commentaries on themes from the book, with Panelist 2 exploring the role that stigma plays in both pain and addiction medicine and Panelist 3 focusing on the use of shared decision making in contexts of uncertainty. To help promote audience engagement, we propose to use the ASBH listserve at the end of the summer to announce the Book Club and a call for short (5 minute), prepared commentaries on the book. With the remaining 40 minutes after the prepared portion of the panel, we will invite audience members selected from this call to deliver their prepared comments, which may include questions or challenges for any of the three panelists, after which we will take question from the rest of the audience.
1:15 pm-2:45 pm | Location: Room 316

Addressing health disparities is a policy priority in the United States. In addition to prioritizing disparities between gender, racial, and ethnic groups, U.S. policymakers and researchers also prioritize disparities related to disability status. Although great efforts have been directed towards a better understanding of health disparities over the past several decades, some have lamented that this progress has been slowed by the lack of shared conceptual terminology. The attempt to standardize health disparities research terminology, however, is complicated by the normative assumptions underpinning and motivating all efforts to measure and address health disparities. This presentation will consider the unique ways these complications manifest in the context of disability-related disparities. After introducing a distinction between health differences, health disparities, and health inequities, it will describe some of the ethical justifications for caring about health disparities in the first place. Disability status is a morally suspect class in the context of American society and history. Thus, it warrants attention from health disparities researchers. Yet, the complicated relationship between disability and health raises unique difficulties for the measurement of disability-related health disparities. These difficulties are further complicated by the lack of consensus over what justice requires us to provide to people with disabilities. As research increasingly focuses on disability-related disparities, these difficulties will need to be acknowledged and addressed.
1:15 pm–2:45 pm | Location: Room 302
Hyde and Go Seek Funding: Resource Allocation by Grassroots Abortion Funds
Ariella Messing, PhD Candidate

Abortion is the only medical procedure that the federal government is prohibited from funding and that the Affordable Care Act explicitly permits states to ban health insurers from covering. The compounded effects of federal and state policies restricting insurance coverage of abortion and regulating the provision of abortion care have created significant financial obstacles to the constitutionally protected right to abortion; low-income women are often unable to access abortion care. In response, communities have created abortion funds, locally-run grassroots organizations that provide financial and logistical assistance to people who need help accessing abortion care. Over 70 autonomous funds have developed organically in response to their local environments; while all funds may share the same goals, their priorities and approaches often differ. This exploratory study describes the variation in abortion funds and examines the ethical values and principles that drive organizational decisions. Data was collected using in-depth semi-structured interviews with a purposive sample of 33 abortion fund volunteers and staff at 26 abortions funds across the United States. Topics covered in the interviews include the fundraising process, the values that guide fund allocation decision-making in the context of scarcity, and case management structures, among other process and organizational decisions and rationales. This presentation examines the themes that arose across funds in different geographical and political contexts and the ethical implications of relying on volunteer-based grassroots funding for the provision of a basic health care service.
1:15 pm–2:45 pm | Location: Room 302 (Same session as directly above)
Reproductive Injustice at the Border
Ariella Messing, PhD Candidate, Rachel E. Fabi, PhD; Joanne Rosen, JD

Recent news reports from the U.S.-Mexico border have detailed alarming stories of public health and ethical violations by the current administration: the deaths of immigrant children in U.S. custody; unaccompanied minors prevented from accessing legal and safe abortions; stillbirths among pregnant detainees; infants torn from their mothers by Customs and Border Protection agents. These stories are often reported in isolation, painting a picture of individual incidents of abuse, or of standalone policies. In this presentation, we examine the recent changes in immigration policies that have systematized this mistreatment, including restrictions on abortion access, unsafe treatment of pregnant immigrants, and the separation of families in immigration detention. When considered through a traditional reproductive rights framework, the Trump administration’s “pro-life” or “pro-family” approach to undocumented minors who seek to terminate their pregnancies may seem at odds with attempts to deny appropriate prenatal care to undocumented adults who wish to continue their pregnancies or with the practice of separating families at the border.
In this presentation, we introduce the Reproductive Justice framework and then demonstrate how these policies and practices violate all three primary tenets of Reproductive Justice: the right to have children, the right not have children, and the right to parent children in safe and secure environments. We argue that when analyzed within this conceptual framework, these policies can be seen as components of a single, targeted strategy to control the reproductive autonomy of vulnerable immigrants.
3:00 pm- 4:00 pm | Location: West Atrium
Against Principled Thresholds: A Conventionalist Defense of Abortion

There is a moral presumption against killing an entity with full moral status (FMS). Those opposed to abortion often object that defenses of the moral permissibility of abortion cannot identify a principled threshold at which point fetuses acquire FMS. While some attempt to avoid this conclusion or even bite bullets in response, I offer a different reply: defenders of the moral permissibility of abortion should embrace the claim that there isn’t a principled threshold for FMS. My argument for this claim turns on the observation that in some contexts, the difficulty in identifying principled thresholds for some to have rights against others is evidence that, at the margins, there are no principled thresholds for the possession of these rights. Instead, such thresholds have to be established conventionally or through law. I illustrate this point by considering difficulties in identifying thresholds for property rights, difficulties that count in favor of the claim that boundaries of property rights have to be defined conventionally or legally. Defenders of the moral permissibility of abortion should take a similar tack. Questions about when some entity acquires FMS is a question about boundaries, albeit concerning rights that accompany FMS rather than rights that accompany ownership. The difficulty in identifying boundaries for FMS is evidence that there is no principled threshold for FMS. Instead, the boundaries for FMS have to be established conventionally or legally. Accordingly, it is unconvincing abortion is impermissible on the grounds that liberals cannot identify a principled threshold for full moral status.
4:15 pm-5:45 pm | Location: Room 304-305
Making Decisions with Parents about Chronic Mechanical Ventilation for their Children: Are We Helping or Harming the Dialogue?
Nicholas A. Jabre, MD, MS; Benjamin Wilfond, MD; Laura Sterni, MD; Renee Boss, MD, MHS


The decision to initiate or forgo chronic mechanical ventilation in a child with prolonged respiratory failure is complex and profound. Clinicians and bioethicists may struggle to weigh the implications of each choice, especially when death is a possible outcome. Guidance toward initiating chronic mechanical ventilation places families without adequate supports at risk of financial strain, social isolation, or psychological distress. Alternatively, guidance toward withholding ventilation risks alienating families who are singularly focused on their child’s survival. Deepening our understanding of how parents perceive interactions with clinicians while deliberating chronic mechanical ventilation could help us engage them in a more personalized manner that better addresses their individual goals. We conducted a multi-center, qualitative study examining parents’ experiences of decision-making surrounding chronic mechanical ventilation. We interviewed 40 parents across three regions in the United States from a variety of socioeconomic and familial backgrounds who made a decision to either initiate or forgo chronic mechanical ventilation for their child within the past three years. Here, we report the experiences of those parents and whether their interactions with clinicians during the deliberation process were perceived as helpful or harmful. Preliminary results show that parents’ individual values and viewpoints regarding life with mechanical ventilation heavily shape their approach to this high-stakes decision. Healthcare professionals should practice intentional curiosity about the unique attitudes and perspectives of parents in order to provide quality guidance that is both patient and family-centered.
–Sunday, October 27–
Concurrent Sessions – 8:00 am-9:00 am | Location: Room 310-311
The Ethical, Legal, and Social Implications of Vaccinomics in the United States
Jennifer E. Gerber, MSc; Janesse Brewer, MPA; Andrea Sutherland, MD, MSc, MPH; Rupali J. Limaye, PhD, MPH, MA; Gail Geller, ScD, MHS; Jeff P. Kahn, PhD, MPH; Daniel A. Salmon, PhD, MPH


objectives: Vaccinomics may use genomics to make vaccines safer and more effective, increasing resilience to vaccine preventable infections. Public values around the ethical, legal, and social implications (ELSI) of vaccinomics were explored. methods: Adults ≥18 years-old attended discussion groups in Boulder, CO and Baltimore, MD in 2018. 95 participants (64% female) were recruited through community organizations and schools, approximating the sociodemographic distribution of each city. After a short animation introduced vaccinomics, participants were randomly assigned to 1 of 7 nested discussion groups. results: Participants generally supported the use of genomics to enhance the safety and effectiveness of vaccines. Ethical issues regarding vaccinomics included privacy/confidentiality, discrimination based on genetic information, prevention of adverse reactions, and justice. Vaccinomics-related ELSI concerns varied by age and trust in industry, government, medicine, and science. Fear of increased health insurance rates or losing coverage due to genetic information discovered through vaccinomics was higher among older versus younger participants. Participants trust government to implement vaccinomics more than industry. Participants who experienced or witnessed an adverse event following immunization (“remembrance”) expressed greater interest in vaccinomics’ potential to personalize vaccines. Participants in both cities recalled historical cases of African Americans’ rights and medical research ethics being violated. They worried vaccinomics would not be applied in ways that benefit vulnerable populations, contributing to stigmatization of marginalized communities. conclusion: These findings are informing the development of a nationwide online survey of vaccinomics-related ELSI concerns and support for personalized vaccines and schedules, overall and by level of vaccine confidence, among U.S. adults.
11:00 am-12:30 pm | Location: Room 320
Disrespectful Language in Prenatal Records
Marielle S. Gross, MD, MBE; Mary Catherine Beach, MD, MPH


Language within medical records may reflect clinicians’ biases and convey stigma about patients from one clinician to another. Women may be especially vulnerable to biases and judgment from clinicians during pregnancy, a time of frequent healthcare engagement about morally-fraught reproductive health. We conducted qualitative linguistic analysis of the history and physical (H&P) from 100 randomly selected women admitted for delivery at our academic center in 2017 to characterize language in prenatal records that may convey disrespect for pregnant women.
With thematic analysis, we identified three types of disrespect: objectifying women’s bodies, testimonial injustice, and signaling the “bad mother.” Women’s bodies are objectified by gratuitous, inappropriately intimate details (‘multiple gushes soaking her underwear and trickling down her leg’). Testimonial injustice occurs when subjective experiences, especially of pain, are trivialized and discredited by juxtaposing patient testimony with “objective data” (‘patient reports unbearable contractions, but cervix remains unchanged.’) Finally, clinicians evoke the “bad mother” by highlighting nonadherence (‘only seen in clinic once’ or ‘but declines genetic screening’), questioning judgment (‘aware of⋯increased risk of maternal and fetal morbidity and mortality, still desires trial of labor’), and implying poor self-care (‘patient has no idea when last pap smear was done’).
Prenatal records manifest disrespect for, and often insidiously mock, pregnant women. We discuss textual cues that may help identify disrespectful language, and potential strategies for preventing healthcare providers from codifying and propagating prejudice.
Seminar Series
Remembering Daniel Callahan
By Ruth Faden
It is with profound sadness and a deep sense of gratitude that we mark the passing of Daniel Callahan, who died on Tuesday, July 16th, just two days shy of his 89th birthday.
Callahan was without question one of the founders and intellectual giants of the field of bioethics. Over the course of an extraordinary career, Callahan wrote 47 books, 17 solo-authored. Nine of his books won national prizes; all took difficult topics to new levels of scrutiny and insight, and many expanded the horizons of bioethics inquiry. Callahan’s most recent book, for example, The Five Horsemen of the Modern World (Columbia University Press, 2016) explored climate change, food, and water as well as chronic illness, and obesity.
In the 1970s and 80s, when I was coming of age in bioethics, it is no exaggeration to say that Dan defined much of what was, and still is, the best of bioethics. His commitment to interdisciplinary inquiry, to rigorous engagement with opposing viewpoints, and to seeking solutions to complex problems was like a magnet to me and others that were looking for a way to make a difference in ethics and public policy.
For many in bioethics, Dan was an extraordinary mentor, advocate and friend who took genuine pleasure in the accomplishments of others. I will never forget one letter I received when I was appointed chair of a presidential committee in the 1990s. It was incredibly supportive and encouraging, a “you can do this” kind of note, signed “an anonymous admirer”. It took me barely a nano-second to deduce that the note was from Dan (who sweetly ‘fessed up in a phone call, replete with more words of encouragement and support).
Dan has inspired so many of us in bioethics in so many ways. A true public intellectual, Dan’s prodigious, probing scholarship remains unmatched in its unique combination of rigor and accessibility. Many of Dan’s insights and arguments will remain vital and vibrant well into the future.
But for all of his extraordinary scholarly accomplishments, Dan Callahan’s legacy will perhaps most be defined by the co-founding and flourishing of the Hastings Center.
At the celebration of the Berman Institute’s 10th anniversary we honored the first recipient of the Harvey M. Meyerhoff Leadership in Bioethics Award. A global civic leader, long-time trustee of the Johns Hopkins University, and member and chair of the Berman Institute Board, Harvey (Bud) Meyerhoff played a major role in the founding and success of the Berman Institute. In establishing the Meyerhoff Leadership Award the intent was to honor people who, like Bud Meyerhoff, exemplify extraordinary leadership, specifically within or in support of the field of bioethics.
The selection committee had no difficulty agreeing on who the first recipient of the Meyerhoff Leadership Award should be.
I can think of no one who better represents the ideals of leadership in the field of bioethics than Dan Callahan. In fact, the story of contemporary biomedical ethics cannot be accurately told without identifying the central role of both Dan and the Hastings Center.
In the late 1960s, Dan foresaw the need for an organization that could engage in systematic intellectual study of the ethical issues raised by the new technological medicine and the broader impact of this new medicine on culture. Serving as the director or president of the Hastings Center for its first 27 years, Dan helped guide the Center from a one-room entity in the basement of his house (supported by a small gift from his mother) into a major center for bioethics scholarship and public engagement.
Today the legacy of Dan Callahan, lies not only in the quality of Hasting Center’s own research projects and of Dan’s own scholarship, but also in the numerous other bioethics scholars and centers that Dan has helped spawn in the United States, Europe, and Asia.
All of us in bioethics are deeply indebted to Dan Callahan. Dan will be sorely missed but his voice and his presence will be ever with us.
Image: By Chip Porter – www.thehastingscenter.org, CC BY 3.0
Stephanie Morain, PhD, MPH
Levi Symposium 2019: Containment: Exploring the History, Politics, and Ethics of Infectious Disease Response in a Post-Genomic World
Cynda Rushton Named to Nurse Researcher Hall of Fame
Berman Institute faculty member Cynda Rushton has been selected for induction into the Sigma Theta Tau International Honor Society of Nursing (Sigma) 2019 International Nurse Researcher Hall of Fame. Professor Rushton was chosen for significant contributions to the nursing profession and her sustained research efforts to improve the care and health of people, specifically in the areas of aging and nursing ethics.
As Anne and George L. Bunting Professor of Clinical Ethics at the Berman Institute and the Johns Hopkins School of Nursing, Rushton, PhD, RN, FAAN, focuses on moral suffering and resilience of clinicians, and designing cultures of ethical practice. In 2014, she co-led the first-ever National Nursing Ethics Summit to prepare a blueprint for nursing ethics in the 21st century, and in 2016, an initiative to help nurses transform morally distressing experiences into moral resiliency.
Her most recent work has been designing, implementing, and evaluating the Mindful Ethical Practice and Resilience Academy (MEPRA) to train nurses challenged with patient suffering, resource allocation, and other ethical situations to respond with integrity. She is author and editor of a new book Moral Resilience: Transforming Moral Suffering in Healthcare (Oxford University Press).
“Ethical practice is the bedrock of nursing, and we are at a pivotal junction in health care that demands that we reorient toward our moral compass,” says Rushton. “My work has been supported by so many peers and mentors along the way, which has helped make this honor a reality. I am humbled and grateful to have been selected as part of this distinguished group of nurses.”
Rushton will be inducted at Sigma’s 30th International Nursing Research Congress in Canada, July 2019.
A version of this article was first published by the JHSON.
Genomics and Society Mentorship Program
Apply: Genomics & Society Mentorship Program
Summer Internship Dates: May 26 – August 3, 2019
Application Deadline: Early February, 2019.
Applicants will be informed of their status by March 1.
6 slots available for Summer 2019 cohort
Open to undergraduate students interested in the ethics of research, clinical care and/or public health, the Johns Hopkins Genomics and Society Mentorship Program begins with a 10-week Summer Internship Program (SIP) through the Johns Hopkins School of Medicine. The Johns Hopkins SIP provides summer research experience to underrepresented minorities, students from economically disadvantaged and underserved backgrounds, and students with disabilities. Through the Mentorship Program, these students will gain experience doing research centered on ethical, legal and social implications (ELSI) of genomics.
Visit the Program webpage for more information and to apply.