Reexamining the Ethical Permissibility of Dual Role Consent
Institutional Obligations for Pragmatic Clinical Trials
Data Sharing in Pragmatic Clinical Trials
New Human Embryo Models Require New Guidelines for Their Ethical Use in Research
When the current guidelines regarding the ethical use of human embryos in research were developed, scientists couldn’t imagine the possibility of stem cell-derived embryo models (SEMs) that recapitulate different stages of embryonic development. But now that SEMs exist, new regulations and policies that permit their ethically appropriate research use need to be developed according to the paper, “Human Stem Cell-derived Embryo Models: Towards Ethically Appropriate Regulations and Policies,” published today in Cell Stem Cell.
“The similarity of the stem cell-derived embryos to natural embryos raise many ethical questions. It’s not obvious if they should be considered solely as lab models, or as akin to actual embryos in moral status and legal provisions,” said the paper’s co-author Dr. Jeremy Sugarman of the Johns Hopkins Berman Institute of Bioethics. “Ethical issues loom over rapid advances in embryo modeling and we need to take action now to ensure ethically appropriate scientific ingenuity and process.”
Sugarman outlined a number of broad areas with open questions pertaining to the use of SEMs in research, including:
Technical
- How similar are SEMs to natural embryos? Can they develop a brain, or be implanted?
Ethical
- Do SEMs have moral status? Is it ethically appropriate to derive SEMs? To use them for research?
Regulatory
- Do existing laws, policies and legal definitions apply to SEMs? Are existing laws sufficient to regulate them? Do existing oversight bodies have authority over SEM research? Are new laws needed?
Sugarman points out that the 14-day rule that forms the cornerstone of many current embryo research policies and regulations, not permitting their use 14 days after fertilization, is complicated in regard to SEMs since no fertilization event exists from which the counting of days can begin.
“Existing legal definitions of ‘human embryos’ should be adapted to clarify which current provisions apply to SEMs and appropriate oversight must be established for this research,” said Dr. Sugarman.
“It is crucial that the scientific community continues to promote widespread discussion of experimental possibilities in order to inform the public about latest advances in this field, apprehend societal concerns, and propose specific guides for researchers. This kind of leadership is all the more needed as research progress dares human imagination, confounds ethical intuition and challenges the application of existing policies.”
How Abortion Restrictions Can Influence Research
In the year since the Supreme Court’s decision in Dobbs eliminated the nationwide right to abortion, consequences of heightened restrictions — including increased maternal morbidity and mortality and deepening socioeconomic and racial inequities — have quickly come into view. Less apparent have been the ethical, legal, and practical implications these restrictions have for research involving people who could become pregnant during research and research staff.
In the paper, “Ethical research when abortion access is legally restricted,” published today in Science, a group of researchers led by Dr. Jeremy Sugarman, MD, of the Johns Hopkins Berman Institute of Bioethics say limited access to abortion can pose risks to clinical research participants, potentially compromising the scientific and social value of some research.
“Ambiguous abortion laws and fear of criminal prosecution raise profound concerns and could deter those who might become pregnant from participating in clinical research,” said Sugarman, the Harvey M. Meyerhoff Professor of Bioethics and Medicine at the Berman Institute and Johns Hopkins School of Medicine.
“Those contemplating the development and implementation of policies pertaining to abortion should also consider the potential negative impact on the ability of researchers to advance science that can improve the health and well-being of those who are or may become pregnant and their fetuses.”
The paper outlines a number of implications for clinical research arising from new laws restricting abortion passed in almost half of U.S. states since the Dobbs ruling. For example, participants might be unable to legally obtain an abortion to mitigate harms resulting from research. Alternatively, a pregnancy test required for participation might document an early pregnancy, placing the participant at risk of legal action if the pregnancy results in an early miscarriage and would otherwise have gone unnoticed.
Clinical research staff also might face legal risks, such as prosecution for referring a participant to an abortion provider out of state. And research could be compromised, both by people hesitating to enroll and by leaving researchers hesitant to obtain rigorous data on pregnancy.
The paper also includes points to consider for researchers as they plan clinical studies in the post-Dobbs environment. In research site selection and management, this includes making provisions for legal abortion access for participants. In study design and implementation, points include safeguarding participant confidentiality, obtaining informed consent that includes risks involved with legal restrictions on abortion, and ensuring that institutional review boards have made determinations related to risks or issues associated with legal abortion.
“Stakeholders involved in research with participants who could become pregnant should explicitly consider the points in the paper, both to minimize the risks to participants and staff and to help safeguard the scientific and social value of research,” said Sugarman. “If on careful examination it seems implausible to safely conduct the proposed research at a particular site, consideration should be given to conducting the research elsewhere.”
JHU Collaborates with Addis Ababa University to Launch Research Ethics Master’s Program

In January 2023, faculty and staff of the Johns Hopkins University-Addis Ababa University Research Ethics Training Program (JHU-AAU RETP) gathered in Addis Ababa, Ethiopia, for a symposium and launch of the Research Ethics Specialty Track within the AAU Master of Public Health (MPH) program.
Over the past three years – with support from the U.S. National Institutes of Health, Fogarty International Center (Grant # R25TW001604) and the leadership of Dr. Adamu Addissie (AAU) – the JHU-AAU RETP has focused its efforts on curriculum development and approvals; strengthening capacity of AAU faculty to teach new courses in bioethics through training and mentorship; raising awareness for bioethics and research ethics through outreach, public seminars and creation of a MPH-wide Responsible Conduct of Research (RCR) course; engaging government leaders in Ethiopia to support ongoing efforts to advance research ethics networks and policies; and piloting of the new MPH Specialty Track.
“We are delighted to have this opportunity to work together to generate the first bioethics-related Master’s training program in Ethiopia,” said Joseph Ali, faculty of the JHU Berman Institute and Bloomberg School of Public Health and co-director of the JHU-AAU RETP. “The success of the program depends greatly on the vision and support of key leaders at AAU and Nationally, and the dedication of AAU and JHU faculty and staff. We are thankful for the support and commitments.”
The official program launch in January celebrated these formative efforts, shared emerging needs and opportunities for bioethics in Ethiopia and the region, and recognized the matriculation of a cohort of nine students into the Master’s program. The event, which was attended by over 60 people, was chaired by Prof. Yeweyenhareg Feleke and included speeches from distinguished guests of the Ethiopian Ministry of Education, AAU administration, and a keynote delivered by Dr. Paulina Tindana (University of Ghana; Accra, Ghana). A panel that included guest speakers Dr. Erisa Mwaka (Makerere University College of Health Sciences; Kampala, Uganda), Dr. Violet Naanyu (Moi University; Eldoret, Kenya), Dr. Yimtubezinash Woldeamanuel (AAU; Addis Ababa, Ethiopia) and Dr. Telahun Teka (National Research Ethics Board; Addis Ababa, Ethiopia) was also organized to share reflections on the future of research ethics in Africa. This was followed by a presentation by Joseph Ali on global efforts to establish mechanisms for benchmarking ethics oversight of health-related research. In the days following, students had the opportunity to begin to share their hopes for their Master’s training and receive feedback from the program and invited guests on early thesis research concepts. Information about local and global bioethics networks and resources were also shared.
In addition to the symposium and meetings with students, the team met with AAU program faculty for a curriculum workshop, and hosted a meeting – chaired by Dr. Andrea Ruff (JHU Bloomberg School of Public Health) – which gathered Institutional Review Board (IRB) leaders from across Addis Ababa for a discussion on IRB capacity strengthening and networking.
While the COVID-19 pandemic has challenged international collaborative training programs, through creative adaptations, personal commitments and supportive institutions, the JHU-AAU-RETP has established a foundation for what it hopes will be decades of successful bioethics training to advance high quality research and practice. On the final evening, while the team gathered for dinner, Dr. Tindana offered the well-known proverb: “If you want to go fast, go alone. If you want to go far, go together.”
Events throughout the week were coordinated by Lealem Wagaw (AAU) and Elise Wilson (JHU). For more information about the JHU-AAU RETP, please contact [email protected] or [email protected].

Join the Berman Institute at ASBH 2021

The Berman Institute will be well represented at the 23rd annual meeting of the American Society for Bioethics and Humanities (ASBH), with a group of faculty, fellows, and students scheduled to present online.
View the full schedule and summaries of all Berman Institute presentations.
 |
You can also follow us on Twitter: #ASBH21, featuring our @bermaninstitute, @aregenberg, @kahnethx, @tnrethx, and more. |
Addressing Social Justice Through the Lens of Henrietta Lacks
Among the many disruptions of the pandemic, one particular disappointment was the cancellation of the in-person annual meeting of the American Society for Bioethics and Humanities (ASBH), scheduled for Baltimore and set to coincide with the Berman Institute’s 25th Anniversary Celebration and the centennial of Henrietta Lacks’s birth. Yet despite the switch to a virtual format, the Berman Institute was able to host a plenary session that was the talk of the meeting and continues to reverberate.
“Social Justice and Bioethics Through the Lens of the Story of Henrietta Lacks,” was moderated by Jeffrey Kahn and featured Ruth Faden as a panelist. She was joined by Henrietta Lacks’s granddaughter, Jeri Lacks, architect Victor Vines, and Georgetown University Law Center bioethicist Patricia King.
Faden began the session by providing an overview of the Henrietta Lacks story, famed in the context of structural injustice.
“The structural injustice of racism defined in pretty much every way how this story unfolded,” she said. “What is wrong about what happened to the Lacks family engages every core element of human well-being. There were assaults on the social basis of respect, and of self-determination, on attachments, on personal security and on health. Mrs. Lacks and her children were poor Black people in a segregated world in which the most profound injustices of racial oppression were daily features of their lives.”
Faden was followed by Jeri Lacks who expressed the importance of continuing to let the world know about her grandmother’s story.
“Her cells were used to develop the polio vaccine and to treat HIV, and in creating in vitro fertilization. She is a person who continues to give life, and to preserve life,” said Lacks. “No matter what your race, your age, your social circumstances, she continues to improve your life.”
Victor Vines, an architect who was part of the architect team leading programming and planning for the National Museum of African American History and Culture and led the feasibility study for what will be Johns Hopkins University’s Henrietta Lacks Hall, spoke next about addressing racial injustice through architecture and design.
“When we started work on Lacks Hall, we didn’t talk a lot about architecture or design. We talked about what that story is that we want to tell through the building. Meeting with the Lacks family was critically important to that,” Vines said. “We had to understand what they went through and what they care about. The building still has to function and house the Berman Institute, so we had to meet their needs. And we discovered a third client, the East Baltimore community. At the end of the day, this building and university reside within that community, and they will be called to embrace this project – or not.”
King concluded the panel with a riveting and wide-ranging discussion that touched upon intersectionality, segregation, the Tuskegee experiments and participation in clinical trials, COVID, race as a social construct, and the role of consent, all within the framework of Henrietta Lacks’s story.
“Our narratives are important and should be thought of as lessons or homework for institutions,” she said. “They not only document the deep distrust we bring to health encounters but also convey relevant aspects of our lives that should be appreciated.”
As the session ended Kahn noted that perhaps it was fortunate the session had been virtual, so the recording “could be shared with others for posterity. I’m not quite speechless, but maybe close,” he said.
Recapping the Berman Institute at ASBH 2020

The Berman Institute will be well represented at the 22nd annual meeting of the American Society for Bioethics and Humanities (ASBH), with a group of faculty, fellows, and students scheduled to present online.
View the full schedule and summaries of all Berman Institute presentations.
 |
You can also follow us on Twitter: #ASBH19, featuring our @bermaninstitute, @aregenberg, @kahnethx, @tnrethx, @DiStefano_MJ, and more. |
Special Events

Plenary: Social Justice and Bioethics through the Lens of the Story of Henrietta Lacks
October 15, 2020
1:15-2:30 p.m.
Join Jeff Kahn, Ruth Faden, Jeri Lacks (granddaughter of Henrietta Lacks), and Patricia King for a panel discussion examining social justice and bioethics through the lens of issues and challenges raised by the story of Henrietta Lacks and the HeLa cell line derived from her cells.
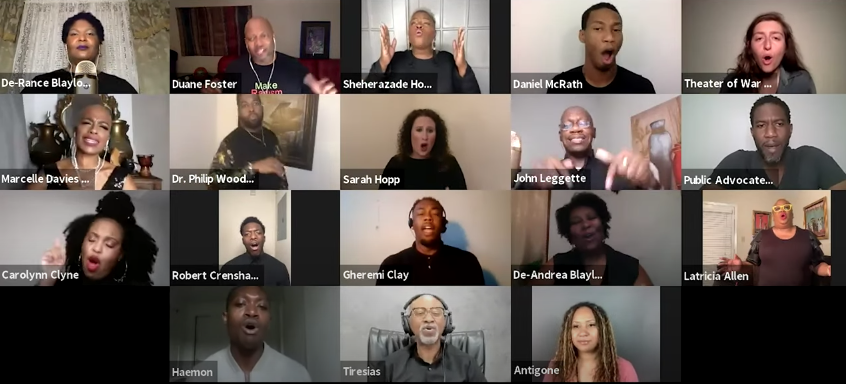
Antigone in Ferguson: Free Online Zoom Performance
October 17, 2020
6- 8:30 p.m.
A groundbreaking project that fuses dramatic readings by acclaimed actors of Sophocles’ Antigone with live choral music culminating in a powerful, healing discussion that will foreground the perspectives of people in Baltimore whose lives have been impacted by racialized police violence and health inequity
Berman Institute Creates Course for Medical Students Disrupted by COVID
The education of students at Johns Hopkins School of Medicine was among the countless disruptions caused when the COVID-19 pandemic first struck in March, as new safety measures suddenly prohibited medical students who would normally have been doing clinical rotations from accessing the hospital and patients. Seeing an opportunity to provide a meaningful and timely alternative, the University’s Berman Institute of Bioethics quickly created and offered a new elective, Ethical and Policy Challenges in the Era of Covid-19: Implications for Clinical Practice, Research and Public Health, that immersed students in independent scholarly research projects studying the pandemic’s impact in real time.
“When I was doing background research, ethics felt like a very academic and philosophical subject,” said Laura Pugh, a third-year medical student with an interest in Internal Medicine. “In the course, I really appreciated the application of ethics and the way it was not just used to explore theoretical ideas, but to bring it into practice and make recommendations for ways things could and should be done in a better way.”
During the course, Pugh conducted two projects related to allocation frameworks for rationing life-saving care. One compared systems for allocation, especially for people with disabilities, and one was creating an intellectual history of changes in thinking about allocation frameworks from the early 2000s to the start of the pandemic.
Read Pugh’s paper, “Disability and discrimination in triage frameworks: a commentary on mathematical approaches to reducing discrimination.”
Each student in the course was paired with a Berman Institute faculty member whose research interests aligned with the student’s. Formal courses meetings occurred (via Zoom) once a week for two hours from mid-April until late May. The majority of the course was the students’ independent research work on their projects, guided by weekly meetings with their faculty mentors.
“Bioethics is not just a theoretical field,” said Gail Geller, the Berman Institute’s Director of Educational Programs, who created the course. “These medical students learned that it’s also a place to do serious, rigorous empirical research projects.”
Students Katie Clark and Megan Hunt teamed to conduct an empirical assessment of healthcare workers’ attitudes about self-infection/immunity passports, as well as a state-by-state comparison of plans to end social distancing. Their paper, “SARS-CoV-2 safer infection sites: moral entitlement, pragmatic harm reduction strategy or ethical outrage?” has been published by the Journal of Medical Ethics.
“I became especially concerned about disparities that came up in some of our projects, and how we could create policies that provide everyone equal access to healthcare, and even augment care for those already facing disparities,” said Hunt. “Particularly in the ventilator project, we found it very enlightening to delve into the principles of how people are justifying medical decision making, deciding who is entitled to what, and what risk we’ll accept for ourselves and for other people.”
Also accepted for publication was Jareatha Abdul-Raheem’s paper, “Re-imagining the Role of School-Based Health Centers during the COVID-19 Pandemic,” which will appear in a forthcoming edition of the Journal of School Health.
Other projects included:
- Qualitative interviews with obstetrical and pediatric providers to assess their views of home vs. hospital births in light of the COVID-19 pandemic;
- Assessing the impact of infection prevention and control policies, in particular visitor restrictions, in an inpatient labor and delivery setting on exacerbating disparities in obstetric outcomes for black women; and
- Reimagining the role of SBHCs in increasing access to care during COVID-19 through expansion of telehealth services, enhancing SBHC-parental communication and engagement, and improving continuity of care through SBHC-community provider partnerships.
“As a future physician, a lot of my experience with ethics is cases, the one-on-one patient perspective. I was rarely thinking about public health on a larger scale, but rather dealing with it on a micro level,” said Abdul-Raheem. “What drew me into bioethics was delving deeply into the health implications of the Covid is affecting everything on a macro level.”
The Berman Institute faculty mentors included two physicians, Megan Collins and Marielle Gross, philosopher Anne Barnhill, public health bioethicist Ruth Faden, and general bioethicist Alan Regenberg.
“The interdisciplinary nature of the elective’s mentors demonstrates how COVID has blurred boundaries in a beneficial way,” said Geller. “Both within the University and between experts in fields like bioethics, medicine, and public health, we’re all working together in new and effective ways.”
Planning How to Allocate and Distribute a COVID-19 Vaccine
Researchers from the Berman Institute of Bioethics have co-authored a new report providing an ethical framework for making decisions about allocation and distribution of a COVID-19 vaccine during the initial period when such a vaccine has first been authorized for use and is still in limited supply.
Released by the Center for Health Security at Johns Hopkins Bloomberg School of Public Health, the report, Interim Framework for COVID-19 Vaccine Allocation and Distribution in the United States, proposes specific tiers of high-priority candidates for receiving a first vaccine based on this framework, including recognizing the contributions of essential workers who have been overlooked in previous allocation schemes:
Tier 1 includes those:
- Most essential in sustaining the ongoing COVID-19 response.
- At greatest risk of severe illness and death, and their caregivers.
- Most essential to maintaining core societal functions.
Tier 2 includes those:
- Involved in broader health provision.
- Facing greater barriers to access care if they become seriously ill.
- Contributing to maintenance of core societal functions.
- Whose living or working conditions give them elevated risk of infection, even if they have lesser or unknown risk of severe illness and death.
The framework is guided by the following ethical principles, which the report authors believe should guide COVID-19 vaccine allocation and help identify more specific policy goals and objectives around vaccine policies:
- Promotion of the common good, by promoting public health while enabling social and economic activity.
- The importance of treating individuals fairly and promoting social equity, for example by addressing racial and ethnic disparities in COVID-19 mortality, and by recognizing the contributions of essential workers who have been overlooked in previous allocation schemes.
- The promotion of legitimacy, trust and a sense of community ownership over vaccine policy, while respecting the diversity of values and beliefs in our pluralist society.
The Berman Institute’s Anne Barnhill, Carleigh Krubiner and Alan Regenberg are among the co-authors, as is former Hecht-Levi Fellow Justin Bernstein, and Ruth Faden contributed.
You can access the new report here.