- Johns Hopkins Berman Institute of Bioethics
- ASBH
- The Berman Institute at ASBH 2020

Join Jeff Kahn, Ruth Faden, Jeri Lacks (granddaughter of Henrietta Lacks), and Patricia King for a panel discussion examining social justice and bioethics through the lens of issues and challenges raised by the story of Henrietta Lacks and the HeLa cell line derived from her cells.
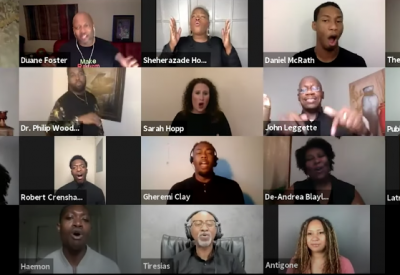
A groundbreaking project that fuses dramatic readings by acclaimed actors of Sophocles’ Antigone with live choral music culminating in a powerful, healing discussion that will foreground the perspectives of people in Baltimore whose lives have been impacted by racialized police violence and health inequity
The Berman Institute at ASBH 2020
The Berman Institute will be well represented at the virtual 22nd annual of the American Society for Bioethics and Humanities (ASBH), with a group of faculty, fellows, and students scheduled to present. The full program is available online.
Preview our diverse presentations below by:
Schedule | Presenter
Wednesday, October 14
Engaging Policymakers on Bioethics Issues
11:00 AM – 2:30 PM EST – ZOOM ROOM 3
PRE-CONFERENCE WORKSHOP (LIVE)
Presenters: Aaron Levine, Debra Mathews, Beth Roxland, Emily Cloyd
This virtual workshop, led by an expert in science communication from the American Association of the Advancement of Science, provides best practices and actionable tools to allow participants to begin strategically engaging with policymakers and support the use of bioethics expertise in the policymaking process. The session starts with an overview of the science and health policy landscape and the role of scientists and bioethicists in the policy process. The workshop introduces best practices for working with policymakers at a local, state or national level, including strategies for accessing and engaging with policymakers. The workshop includes facilitator presentations and individual and small group exercises using virtual breakout groups. Each participant will identify an individual communication goal and develop short messages that will resonate with specific policy audiences. In addition, participants will rehearse an in-person meeting with a policymaker, such as a member of Congress or her/his staff, gaining practice with this important form of policy engagement. Key best practices will be illustrated with examples from ASBH members experienced in engaging with policymakers. Exemplars will include Debra Mathews, who will share lessons learned from her experiences testifying before legislative bodies at the state and federal level and serving as a staff member on the Presidential Commission for the Study of Bioethical Issues, and Beth Roxland, who will draw on her experience as Executive Director of the New York State Task Force on Life and the Law, including her role helping enact the Task Force’s Brain Death Report into state regulations, and as policy analyst and advisor.
Thursday, October 15
Informing Ethical Policy: A Justice-Based Approach to Drug Shortages
11:00 AM – 12:15 PM EST – ZOOM ROOM 8
PANEL PRESENTATION (LIVE)
Presenters: Andrew Shuman, Megan Petry, Douglas Throckmorton, Yoram Unguru
Drug shortages are a national tragedy that represent a public health crisis preventing patients from accessing lifesaving medications. They represent a symptom of our inherently damaged healthcare system in which pharmaceutical companies are perversely dis-incentivized to produce inexpensive generic, but critical drugs. The absence of policies incentivizing quality and increased manufacturing of essential medicines further constrains our broken healthcare marketplace. These consequences of our current governance structure will require legislative and regulatory action. The panel features two practicing oncology doctors, a pediatrician and a surgeon, who are clinical ethicists studying responses and solutions to drug shortages; they will use the recent vincristine shortage as a paradigmatic example, review how we got here, and summarize an ethical framework to potential solutions. A senior administrator of the US Federal Drug Administration (FDA) who has been at the helm of federal responses to drug shortages and helped release a long-anticipated report that contextualized the current state of drug shortages will provide the regulatory perspective. In addition, an investigative counsel from the United States Senate Homeland Security and Governmental Affairs Committee minority staff who produced a report, “A Price Too High: Cost, Supply, and Security Threats to Affordable Prescription Drugs,” will share tangible and actionable legislative solutions. This multidisciplinary group will then discuss how federal policy, regulatory, and legislative changes can be directly informed by a shared framework of responsibilities in a manner that recognizes real-world constraints, but also ensures that patients in need are able to fairly access essential medications.
Linguistic Bias in Language Used by Physicians in Medical Records of African American and White Patients: A Case of Testimonial Injustice?
12:30 – 1:00 PM – ZOOM ROOM 4
PAPER PRESENTATION Q&A (LIVE) – RACE, BIAS, ACCESS, AND DISPARITIES
Presenter: Mary Catherine Beach
Co-author: Marielle Gross
Background. African Americans and women report having medical concerns ignored or downplayed by health professionals. We explored racial/ethnic and gender differences in language suggesting disbelief of patients within medical records.
Methods. We previously identified 3 relevant linguistic features: (1) quotes (e.g.“reaction”); (2) negative words (e.g. claims, adamant); and (3) clausal complements, a sentence construction where the source of information is conveyed by providing key information as a clause (e.g. pt reports that headache started yesterday vs. the headache started yesterday). Although clausal complements are common in medical records and do not cast explicit doubt, we hypothesized that the overall number of sentences with that format may reflect greater doubt. We used natural language processing to identify features in ambulatory internal medicine notes written in 2017, and tested race/gender differences using mixed-effects Poisson regression accounting for clustering within patients and providers.
Results. Our sample included 7,986 notes written by 164 clinicians about 1890 patients (801 Black women, 630 Black men, 254 white women, and 205 white men). Notes written about Black patients had a greater number of quotes (p=0.039), negative language (p=0.006) and clausal complements (p<0.001). Analyses by gender were mixed: notes about female vs. male patients did not differ in negative language but had a greater number of quotes (p=0.002) and fewer clausal complements (p<0.001). There were no significant interactions between race and gender.
Discussion. African American patients may be subject to systematic bias in physicians’ perceptions of their credibility. Further studies are needed to understand these phenomena.
Social Justice and Bioethics through the Lens of the Story of Henrietta Lacks
1:15 – 2:30 PM EST – ZOOM ROOM 1
PLENARY
Moderator: Jeffrey Kahn
Presenters: Ruth Faden, Jeri Lacks, Victor Vines, Patricia King
Social justice and bioethics have never been more important than in our current times. This plenary session will feature a moderated panel discussion examining social justice and bioethics through the lens of issues and challenges raised by the story of Henrietta Lacks and the HeLa cell line derived from her cells. Topics include structural injustice; the perspective of members of the Lacks family; a retrospective on law, social justice, and bioethics from the release of the Belmont Report in 1979 to the present; and a discussion of how architecture and design can represent legacy and naming. An audience Q&A will follow the panel presentations.
Responding to Treatment Refusal, Respecting Pluralistic Values and Retaining the Child’s Right to Flourish
2:45 – 4:00 PM EST – ZOOM ROOM 2
PANEL PRESENTATION (LIVE)
Moderator: Yoram Unguru
Presenters: Yoram Unguru, Amy Caruso Brown, Erin Paquette
Guided by the best interest standard, parents are granted wide latitude in making decisions for their children. Children and/or parent(s) may disagree with their physician’s treatment recommendation. There are, however, limits to the decisions society will allow children and parents to make on behalf of their children. Refusal of life-saving treatment for a potentially curable childhood cancer provokes strong emotions for members of the healthcare team often resulting in frustration and at times, moral distress. It is up to individual physicians to decide whether to report treatment refusal (TR) and initiate medical neglect proceedings. Among childhood cancer providers, evidence suggests that a sizable minority of pediatric oncologists opt not to report. Moreover, many physicians are more willing to accept requests for TR when the prognosis is “poor” and the parent and older child have made a mutual decision. In deciding whether to report, it is less clear whether physicians rely upon a specific survival threshold and how they grapple with prognostic uncertainty or burden and length of treatment. Similarly, how does physician bias and/or discrimination influence reporting decisions, specifically, patient/family factors such as race, culture, educational level, personality, and reason for TR (religious objection, desire for complementary/alternative therapies)?Viewed through the lens of “justice and flourishing” we consider the role for pluralistic values/morals in cases of TR. To frame the discussion and highlight many of these themes, we will present an actual case of an adolescent with curable cancer who along with his parents refused recommended care.
“Safe Harbor” for Deidentified Data as a Barrier to Ethical Learning Health Systems
4:15 – 4:45 PM EST – ZOOM ROOM 8
PAPER PRESENTATION Q&A (LIVE) – PATIENT DATA AND PATIENT PRIVACY
Presenter: Marielle Gross
To promote learning while minimizing privacy risks in the U.S., deidentified health data is neither restricted by HIPAA nor governed by Common Rule human subjects’ protections (AKA “safe harbor”). Meanwhile, the ethical framework for learning health systems (LHS) proposes both that clinical care and research no longer be treated as ethically distinct, and that patients are obligated to contribute to learning, primarily by allowing research on health data generated during clinical care. We argue that patient-centered outcomes research, which involves questions and outcomes “meaningful and important to patients and caregivers,” under current “safe harbor” for deidentified data may undermine distributive justice, respect for persons and beneficence. First, deidentification intentionally eliminates accountability to patients whose data are studied, making research easier, but also precluding patients from direct clinical benefits or recognition. This contradicts just distribution of research benefits and burdens, especially when findings are timely or personally significant, exacerbated by background conditions in which access to healthcare is not universal. Further, lack of human subjects’ protections may functionally dehumanize deidentified patients by allowing commodification or monetization of inherently intimate data—data often obtained via invasive examinations performed under auspices of advancing individuals’ health interests—without consent. Finally, deidentification’s beneficent intentions are challenged by artificial intelligence coupled with increasing clinical, genomic and biological data, and by systematically emphasizing siloed, incomplete datasets. Ultimately, deidentification may impede flourishing of a just LHS by maintaining the distinction between patient and research subject, neglecting duties of care to both, and preventing seamless treatment, learning and feedback.
Covid-19 as Hereditary Illness? Social and Ethical Implications of the Blurry Boundary between Infectious and Genetic Disease
4:15 – 4:45 PM EST – ZOOM ROOM 16
PAPER PRESENTATION Q&A (LIVE) – NEW THINKING FOR A NEW NORMAL
Presenter: Rebecca Wilbanks (BRIDGES)
Genetic/ hereditary diseases and infectious diseases are normally thought of as distinct categories of pathology. Yet as current research into genetic factors corresponding with severe cases of Covid-19 indicates, a person’s genetic inheritance is likely to play a role in the likelihood of infection and course of illness. While such recent research into host-pathogen interactions seems to blur the boundaries between infectious and genetic diseases, the history of medicine demonstrates that the effort to assign diseases into specific categories based on their mode of transmission has always been contested and unstable, as researchers and publics have attempted to weigh the roles of contagion, constitution, and environment. How might social conceptions of Covid-19 shift if host genomics initiatives identify predictive markers of illness, and what ethical questions does such research raise? This paper looks to history to answer these questions, examining three diseases that have moved across different frameworks of causation at different times: tuberculosis, AIDS, and hereditary colorectal cancer. These examples are used to explore how a categorization as hereditary or infectious influences the social conception of a disease as well as the stigma associated with it, and what happens when new research shifts or complicates our understanding of disease etiology. Returning to Covid-19, the paper will conclude by suggesting a systems approach that integrates multiple modes of causation including the social determinants of disease, such as poverty and racism, that affect morbidity and mortality.
Access the pre-recorded paper presentation here.
Contemporary Ethical Controversy in Fetal Therapy: Innovation, Research, Access, and Justice
5:00 – 6:15 PM EST – ZOOM ROOM 16
PANEL PRESENTATION (LIVE)
Moderator: Naomi Laventhal
Presenter: John Lantos, Armand Antommaria, Marielle Gross
Maternal-fetal care centers offer an increasingly broad range of fetal therapies to improve survival and quality-of-life for infants with congenital anomalies. Most interventions are not well-studied, raising ethical issues for clinician-scientists, patients, policy makers, and research institutions. Should these interventions be classified as experimental and only offered under IRB approved protocols? How should women be counseled regarding ultrasound findings for which fetal therapies may offer benefit when they are neither widely available nor standard-of-care? To what lengths should a “good mother” go for her baby? How should fetal interventions be studied in the context of regulatory complexity, competing stakeholder interests, diagnostic and prognostic uncertainties, and small patient populations? We explore some of the pressing and unresolved ethical questions about fetal intervention research.The moderator, a neonatologist, bioethicist, and expert in antenatal consultation, will introduce the salient issues using an illustrative case. A pediatrician and expert in neonatal and research ethics will examine in the tension between innovation and research, with attention to how families and clinicians make decisions to pursue these interventions. Another pediatrician bioethicist will explore when innovative therapy should be subject to systematic evaluation and what types of experimental designs and eligibility criteria are appropriate with attention to equitable access to potential treatments. An OB/GYN bioethicist will examine ethical, legal, and social dimensions of care for pregnant women in the face of emerging, paradigm-shifting, surgical and genetic technologies, disparities, legal constraints to reproductive choice, and sociological context of idealization/judgement of pregnant women qua mothers.
Is Rarer Fairer? An Interactive Drama Explores Justice and Resources-Intensive Innovative Therapies for Rare Diseases
5:00 – 6:15 PM EST – ZOOM ROOM 10
PERFORMANCE AND EXHIBITION (LIVE)
Panel: Lynn Bush, Christine Mitchell, Karen Rothenberg, Robert Truog
This performance session premieres a multi-case drama, mock attendee advisory committee, and family video to actively engage the diverse ASBH community in conversations exploring notions of Justice and Fairness in the context of resource-intensive new interventions for rare genetic diseases. Both the play’s dialogue and two video excerpts illuminate the complex ethical landscape of translational medicine to critically examine implications, interests, perspectives, and tensions between individuals, families, communities, and our pluralistic society-at-large. Questions abound, such as who should pay the exorbitant costs for the development and administration of innovative therapies, how does an institution decide the inclusion criteria for access to the therapy, and should it make a difference whether the resource allocation may only benefit a small number even within the rare disease community? The twenty-minute play sets the stage to enhance reflective ethical consideration of justice issues and foster lively inter-professional discourse. After the performance, the multidisciplinary bioethicist-presenters (PhD; RN; JD; MD) and nation-wide troupe of distinguished bioethicist-actors, share insights based on their character’s role and own experiences. Broad audience discussion is elicited; participants are also encouraged to provide feedback regarding this narrative-genomic pedagogy as an approach for ethics education as well as interprofessional and community engagement. Attendees are then engaged in a mock Ethics Advisory Committee tasked with voting on select decision-making processes to guide policy for this fictional institution. Lastly, we present and discuss two powerful excerpts from a video shared by a family who has a child with a rare genetic condition.
Friday, October 16
Building Diversity in ELSI: Reflections from Training Program Directors
10:45 – 11:45 AM EST – ZOOM ROOM 11
ELSI AFFINITY GROUP MEETING
Moderator: Aaron Goldberg
Panelists: Debra Mathews, James Tabery
Behind the Curtain: An Ethical Analysis of Menstruation Tracking App Data
12:00 – 12:30 PM EST – ZOOM ROOM 1
PAPER PRESENTATION Q&A (LIVE) – MOBILE HEALTH APPLICATIONS, HEALTH INFORMATION SYSTEMS, AND HEALTHCARE METRICS
Presenter: Amelia Hood
Co-authors: Marielle Gross, Bethany Corbin, Margot Kelly-Hedrick
The recent explosion of digital health applications includes emergence and growing popularity of smartphone apps for women’s health, most commonly menstruation tracking apps. Meanwhile, monetization of women’s sensitive health data has led to public outcry and ethical concerns. Here, we explore menstruation apps as a case study of the creation of health data outside of traditional healthcare contexts from a medico-legal and justice perspective. Menstrual apps normalize a new frontier for ubiquitous data surveillance, gathering health-relevant data outside of the traditional healthcare contexts where it may not be afforded appropriate legal or ethical protection. App companies provide “free” services and then generate profits from user data by selling targeted advertisements for health-related products and healthcare services. Many health apps raise concerns regarding their effectiveness for promoting health— The services offered by the app, namely ovulation and fertility prediction, have been found to be inaccurate, sometimes with life-altering consequences. For example, the Natural Cycles app, the first app to have FDA clearance for its contraceptive algorithm, enticed many women to utilize it as their sole birth control method, but notoriously failing, with many unintended pregnancies resulting. We identify and discuss two primary concerns: 1. Menstrual apps impersonate healthcare without appropriate expertise, oversight or fiduciary duties. 2. Menstrual apps, largely funded and designed by men, commodify women’s bodies, treating their reproductive systems as a source of profit, ultimately exploiting their asymmetric power by systematically taking more value (women’s data and role as consumers of advertisements) than they provide (app services).
Access the pre-recorded paper presentation here.
Advancing Ethical HIV Research in Pregnancy: Guidance from the PHASES Working Group
2:15 – 3:30 PM EST – ZOOM ROOM 13
PANEL PRESENTATION (LIVE)
Moderator: Kristen Sullivan
Presenters: Kristen Sullivan, Annie Lyerly, Ruth Faden, Maggie Little
Numerous barriers to the inclusion of pregnant women in biomedical research have resulted in profound and consequential evidence gaps about how to safely and effectively treat and prevent HIV and co-infections during pregnancy. This puts women and the children they bear in harm’s way. Without timely pregnancy-specific data on safety, drugs may be used that carry unacceptable risks to the fetus or pregnant woman. Additionally, in the absence of pregnancy-specific data on how drugs interact with the distinctive physiology of pregnancy, pregnant women may be given doses that are too low or too high. Perhaps most profoundly, delays in gathering evidence can mean a delay in pregnant women’s access to next-generation drugs — and the important benefits they may entail over older drug options. In order to address this pressing need and advance timely, responsible research with pregnant women, the Pregnancy and HIV/AIDS Seeking Equitable Study (PHASES) Working Group – an international, interdisciplinary, and inter-sectoral group of 26 individuals from across the HIV research and advocacy community – developed concrete ethics guidance, informed by extensive stakeholder engagement. This panel presentation will: 1) orient participants to the harms created by the evidence gap for pregnant women in HIV/co-infection prevention and treatment; 2) describe PHASES’ empirical research that informed the Guidance; 3) articulate the ethical foundations of the Guidance, and 4) share the 12 recommendations in the Guidance, directed to multiple stakeholders, which aim to secure better, earlier, and more systematic evidence on safely and effectively treating and preventing HIV and co-infections during pregnancy.
The Ethics and Governance of Digital Contact Tracing Technologies for Pandemic Response
4:45 – 6:00 PM EST – ZOOM ROOM 15
PANEL PRESENTATION (LIVE) – PANDEMIC, POLICING, AND DIGITAL DATA
Presenter: Amelia Hood
Co-authors: Jeffrey Kahn, Joseph Ali, Anne Barnhill, Anita Cicero, Katelyn Esmonde, Brian Hutler, Alan Regenberg, Crystal Watson, Matthew Watson
As public health professionals around the world work tirelessly to respond to the COVID-19 pandemic, it is clear that traditional methods of contact tracing need to be augmented in order to help address a public health crisis of unprecedented scope. Rapidly developing digital technologies may aid public health surveillance and containment strategies for this pandemic and become part of the larger toolbox for future infectious outbreak prevention and control. The Johns Hopkins Project on Ethics and Governance of Digital Contact Tracing Technologies carried out an in-depth analysis of the technology and the issues it raises. Bringing together perspectives from bioethics, health security, public health, technology development, public policy, and law, we present clear and detailed guidelines to help manage the responsible creation, implementation, and application of digital contact tracing – prioritizing alignment of technology with public health needs and public values, building choice into design architecture, addressing equity considerations, and capturing real-world results and impacts to allow for continuous learning.
Saturday, October 17
Issues of Privacy and Informed Consent in the Era of Government-Led DNA Profiling Programs
2:15 – 2:45 PM EST, ZOOM ROOM 17
FLASH PRESENTATION Q&A (LIVE) – CONSENT, PARTICIPATION, POWER, AND TRUTH TELLING
Presenter: Sheethal Jose
In 2019, the Trump administration implemented a pilot program to perform “Rapid DNA” testing on immigrants at the US-Mexico border to further justify their policy of family separation. In addition to using these tests to determine the biological relationships between migrant children and their accompanying adults, there are plans to enter the DNA information into the FBI Combined DNA Index System (CODIS) database. Similar government-led forced DNA profiling programs have been implemented in China, where the government has been collecting DNA samples from the detained Muslim Uyghur population to aid law enforcement agencies. The ethical, legal, and social implications (ELSI) of using DNA testing to profile minority populations, especially in the context of law enforcement, are significant. Major ethical concerns include privacy, informed consent, racial discrimination, and social justice issues raised due to the targeting of vulnerable populations. Forcefully subjecting an individual to genetic testing is a gross violation of privacy rights. Although migrants are provided with consent forms before being tested, they are pressured under the looming threat of separation if they do not agree to the DNA test. Obtaining consent under these context is not meaningfully voluntary. This pretense of consent should not be the standard for genetic profiling of minority populations. Rather, we need policies that capture the nuances of informed consent in the context of DNA testing and law enforcement.
Access the pre-recorded flash presentation here.
Should Genomics Play a Role in Clinical Management of High Consequence Infections?
2:15 – 2:45 PM EST – ZOOM ROOM 4
PAPER PRESENTATION Q&A (LIVE) – CLINICAL ETHICS AND EMERGING BIOTECHNOLOGIES
Presenter: Gail Geller
Co-authors: Jennifer Gerber, Brian Garibaldi
We are currently in the midst of a coronavirus epidemic. High-level isolation units (HLIUs) (funded by the U.S. Office of the Assistant Secretary for Preparedness and Response after the 2014-2016 Ebola outbreak) are gearing up to care for patients with COVID-19. Evidence from other high consequence infectious diseases (HCIDs) including viral hemorrhagic fevers, SARS, and novel influenza viruses, suggests that host genomics influences susceptibility to such infections, vaccine and treatment response, and morbidity and mortality. Should HLIUs incorporate genomics in patient care? We surveyed HLIU staff to identify the ethical, legal and social implications (ELSI) of using genomic technologies in this environment. 112 staff representing all 10 HLIUs responded. Over 85% agreed with the use of genomic testing to identify patients at risk for poor outcomes from HCIDs, including treatment non-response, morbidity and mortality. However, 30% strongly disagreed with using genomic information to make treatment decisions. 75% felt that it would be unethical to withhold treatment from patients with a high likelihood of mortality, and more than half felt admission to a unit should not be impacted by genomics. 90% would support confidential employer-based testing of healthcare workers to understand individual staff risk, but 84% felt this information should not inform work assignments. 89% of respondents felt they would benefit from additional education on genomics. Our results highlight ELSI concerns about genomic testing in HLIUs, and can inform future research, workforce privacy/discrimination policies and patient treatment allocation decisions regarding the role of genomic testing of patients and staff in HLIUs.
Access the pre-recorded paper presentation here.
Lazy, Selfish Sluts: Shaming the ‘Bad Mother’ in Prenatal Records
2:15 – 2:45 PM EST – ZOOM ROOM 9
PAPER PRESENTATION Q&A (LIVE) – PATIENT TRUST AND PATIENT SHAMING
Presenter: Diana Mendoza-Cervantes
Co-authors: Marielle Gross, Mary Catherine Beach
Clinicians’ language within medical records may reflect biases and transmit stigma to other providers. Here, we examine the language in the medical records of 100 randomly-selected women admitted in 2017 for labor and delivery reflecting judgements against pregnant women qua mothers and the emergent theme of inappropriate presumption, or accusation, of maternal-fetal conflict. We highlight three subcategories implying woman are harming their fetus by failing to act (neglectfulness), acting in self-interest, and “moral contamination.” We also investigate potential racial and socioeconomic disparities in judgmental language. First, comments imply neglectfulness by emphasizing nonadherence to standard-of-care despite awareness of recommendations (“Has yet to complete 28 week labs,” “knows she should be taking iron, but not taking iron,” or “advanced maternal age, but declines genetic screening.” Second, comments depict patients’ decisions as selfish and threatening fetal well-being. For example, “aware of increased risk of maternal or neonatal morbidity and mortality, but still desires trial of labor,” or “hasn’t paid attention to fetal movements because of painful contractions.” Third, language stressing poor self-care, especially related to substance use or risky sex, may appear out of context or out of proportion to clinical significance, implying morally dubious character unbefitting of the modesty, chasteness or responsibleness of the “good mother” (e.g., history of chlamydia appearing five times in a History & Physical note). This category evokes concern that the mother imposes harm via “contamination” of the “innocent” fetus. We consider the impact physicians’ judgement of women as “bad mothers” may have on health outcomes.
Access the pre-recorded paper presentation here.
Is This Child Dead? Controversies Regarding the Neurological Criteria for Death
4:15 – 5:30 PM EST – ZOOM ROOM 3
PANEL PRESENTATION (LIVE)
Moderator: Lainie Ross
Presenters: Lainie Ross, Armand Antommaria, Margaret Moon
One hundred years ago, patients were simply declared dead when their respiration and circulation ceased. However, in 1968, an Ad Hoc Committee of the Harvard Medical School published a report that provided criteria for determination of death by neurological criteria. The Uniform Determination of Death Act (UDDA), proposed in 1981, added the neurological criteria to the cardio-respiratory criteria and states that “A determination of death must be made in accordance with accepted medical standards.” Professional association have subsequently approved guidelines for determining when individuals fulfill the neurological criteria including apnea testing. While many states have adopted the UDDA’s language, several provide forms of accommodation for individuals who reject the neurological criteria. This regime has come under criticism by clinicians and philosophers who argue that these criteria do not accurately determine when individuals have died and has been challenged by families who wish to continue treatment for their loved ones. In this session, three pediatricians will discuss different aspects of the brain death debate and its implications for children. The first speaker, a pediatrician-philosopher, will argue that informed consent for apnea testing is only needed if families are able to choose the criteria by which their loved ones are declared dead. A second speaker, a pediatrician-religious ethicist, will discuss accommodating differing views of death including whether public insurance should cover continued treatment for individuals who fulfill the neurological criteria. The third speaker, a pediatrician-clinical ethicist-hospital administrator, will discuss the practical challenges of applying well-reasoned ethics policies in complex, modern hospital systems.
Management of Collateral Findings in PCTs
4:15 – 5:30 PM EST – ZOOM ROOM 11
PANEL PRESENTATION (LIVE)
Moderator: Stephanie Morain
Presenters: Stephanie Morain, Debra Mathews, Juli Bollinger
Unanticipated findings pose practical, ethical, and legal challenges for researchers, clinicians, institutions, and patients/research participants. While considerable attention has focused on unanticipated findings in clinical care and explanatory research, these issues have not been broadly considered in the context of pragmatic clinical trials (PCTs). Yet managing these “collateral findings” (CFs) in the context of PCTs is challenging, as many PCTs are done without patient consent and/or awareness of the research being done. Furthermore, the substantial scale of PCTs means that CF management may involve considerable resources and efforts for clinicians and health systems. As NIH invests substantial resources to expanding the use of PCTs, there is a critical need for guidance on PCT-CF management. In this session, we will introduce a conceptual overview and background regarding PCT-CFs, and outline why existing guidance has limited applicability for the PCT context. We will then present recent empirical data from three projects that elicited the perspectives of relevant stakeholders regarding their knowledge, attitudes, beliefs and expectations regarding PCT-CFs. First, we will describe the results of qualitative interviews with 60 key stakeholders involved in the conduct, design, and oversight of PCTs, including researchers, clinicians, IRB members, and health system leaders. Second, we will describe the results of 9 focus groups with patients, conducted at 3 different sites to reflect geographic diversity. Third, we will present the results of a national survey of the public. We will then engage the audience in a moderated discussion regarding lessons for the ethical management of PCT-CFs.
Taking Justice Seriously in Health Research and Policy in Low-Resource Settings
4:15 – 5:30 PM EST – ZOOM ROOM 8
PANEL PRESENTATION (LIVE)
Moderator: Maria Merritt
Presenters: Maureen Kelley, Danielle Wenner, Maria Merritt
Three presenters will speak for 15 minutes each. Then the moderator will facilitate audience-presenter dialogue for 30 minutes: approximately 10 minutes for each of three learning objectives.1. Justice-based obligations in health research priority-setting. How far do health research priority-setting obligations extend? The presenter will argue that (a) research funders from high-income countries have priority-setting obligations that extend beyond national borders; and (b) such funders have greater obligations to prioritize research questions that address disease burdens in low-resource settings, while epistemic communities in low-resource settings should prioritize local needs. This approach is suggested by understanding how international research institutions condition global citizens’ life chances.2. How do health researchers address structural injustice? To what extent do researchers engage structural sources of vulnerability in low-resource settings, and what are the implications for research ethics practice? The presenter will describe qualitative findings from an international empirical research ethics study involving six “embedded ethics” case studies in low-resource settings where bioethics teams linked within ongoing biomedical research. This talk will discuss cross-site findings illustrating how researchers navigate participant and community needs arising from deeper social, economic, and political injustices. 3. Representing social justice in health-related policy decisions. How can social justice be represented in comparing health policy options under resource constraints? The presenter will describe a sustained example of using philosophical social justice theory and qualitative research to develop user-friendly analytic tools that supplement traditional economic evaluation, with special attention to enabling the use of local data and local capacity at low cost.
Antigone in Ferguson
6:00 – 8:30 PM EST – ZOOM ROOM 1
PERFORMANCE AND EXHIBITION (LIVE)
Panel: Jeffrey Kahn, Gail Geller
Antigone in Ferguson is a groundbreaking project that fuses dramatic readings by acclaimed actors of Sophocles’ Antigone with live choral music performed by a diverse choir, from St. Louis, Missouri and New York City culminating in powerful, healing discussions about racialized violence, police brutality, systemic oppression, gender-based violence, health inequality, and social justice. The project was conceived in the wake of Michael Brown’s death in 2014, through a collaboration between Theater of War Productions and community members from Ferguson, MO, and premiered at Normandy High School, Michael Brown’s alma mater, in September of 2016, and has since toured the country and the world. In light of the uprising and protests catalyzed by the killings of Ahmaud Arbery, Breonna Taylor, George Floyd, Tony McDade, Dion Johnson, and many others; and the disproportionate impact of the COVID-19 pandemic on black and brown communities, Antigone in Ferguson aims to generate dialogue, consciousness, compassion, outrage, understanding, and positive action at this critical moment. This special presentation of Antigone in Ferguson on Zoom will foreground the perspectives of people in Baltimore, Maryland whose lives have been impacted by police brutality, community violence, and the COVID-19 pandemic. Co-presented by Theater of War Productions, the Johns Hopkins Berman Institute of Bioethics, and the Johns Hopkins Program in Arts, Humanities & Health.
Watch a preview and register online.
Sunday, October 18
A Plague of Darkness: Justice, Ethics and COVID-19
11:30 AM – 12:45 PM EST – ZOOM ROOM 9
PANEL PRESENTATION (LIVE)
Moderator: Laurie Zoloth
Presenters: Laurie Zoloth, Jeffrey Kahn, Jeffrey Bishop, Gayman Bennett
COVID-19 emerged from the center of industrialized China in the late winter of 2020 from under the cover of censorship and consistent public denial. While Americans watched, China finally mounted a massive public health trial, unprecedented in the modern era, of a cordon sanitaire around nearly half of the county. It did not work to contain the epidemic, which as of the submission deadline, has just begun community spread in Europe, the Middle East, and the United States. The epidemic pulls questions of justice that bioethics has been debating for decade into the center of the epidemic. The closing of hospitals has left only 62,000 ICU beds with ventilators available for 300 million people; the bulk of citizens do not have sick days, coverage for tests; the ability to stay home from hourly jobs; to stock up on products in large amounts, or have the spare bathroom that CDC in its imaginary, recommends. Our culture is based on the capacity of the poorest to care for the elderly, to cook and serve, yet it is these people who are most vulnerable. The epidemic also displays the tension between libertarian systems of justice and communitarian ones, between social isolation and human gathering, such as religious or political public spaces. We will explore the profound social dynamics of the epidemics from four different perspectives within the claim that it represents the most important challenge to bioethics and humanities in decades.
Aging and Vaccination in the US: A Roadmap for Research, Ethics, and Policy
1:00 – 1:30 PM EST – ZOOM ROOM 8
PAPER PRESENTATION Q&A (LIVE) – RESEARCH
Presenter: Alexandra Ruth
By 2030, one in five Americans will be 65 years of age or older. Older adults are highly susceptible to infectious diseases due to “immunosenescence,” the natural aging process of the immune system that increases risk for and severity of infections. Vaccination of older adults has received relatively little attention in the public health ethics literature compared to childhood vaccination, which has largely been successful through the use of school vaccine mandates and has played a key role in reducing childhood infectious disease burdens. Meanwhile, older adults in the U.S. still experience significant morbidity and mortality from vaccine-preventable diseases such as influenza, pneumonia, and shingles, yet vaccination rates in this population remain low. Factors driving low uptake of vaccines among older adults include lack of a provider recommendation; perceived unimportance of vaccination; lack of enshrined vaccine mandates similar to those that exist for childhood vaccines; and high costs of vaccines that may require cost-sharing, particularly those covered under Medicare Part D. This paper 1) articulates past and present issues in vaccine ethics, financing, and policy specifically relevant to older adults and 2) evaluates the ways in which current vaccine financing, guidelines and R&D priorities are falling short for the burgeoning aging population, using this analysis to identify specific duties and obligations that the public health community has regarding vaccination needs for an aging population. Finally, this paper synthesizes these duties and obligations into an actionable roadmap for addressing gaps in research, ethics, and policy related to aging and vaccination.
Access the pre-recorded paper presentation here.
Perspectives on Physician-Assisted Dying for Persons with Psychiatric Illnesses
1:45 – 3:00 PM EST – ZOOM ROOM 2
DEBATE (LIVE)
Moderator: Marie Nolan
Presenters: Brent Kious, Margaret ‘Peggy’ Battin, Scott Kim, Daniel Sulmasy
Physician-assisted dying, or medical aid-in-dying (MAID), is legal in ten US jurisdictions but only for persons with terminal illnesses (tMAID). In several European countries, euthanasia or assisted suicide, as it is called there, is permitted for persons without terminal illness but with unbearable suffering, including psychiatric illness (pMAID). pMAID remains controversial even in jurisdictions where it is permitted. Moderator Marie Nolan will present background information about MAID in the US and Europe. Speaker 1, a bioethicist and philosopher familiar with MAID in the Netherlands and with historical sources concerning the ethics of suicide, will question whether the suffering of persons with psychiatric illnesses can sometimes justify access to pMAID. Speaker 2, a psychiatrist and philosopher-bioethicist who has conducted research specifically regarding pMAID in the Netherlands, will argue that equality arguments to expand pMAID are flawed and that there are strong policy and clinical considerations against pMAID. Speaker 3, a psychiatrist and philosopher-bioethicist who examines the role of values in medicine, will focus on treatment-refractory psychiatric illness, arguing that it can involve suffering as serious as that in physical illness; hence pMAID is permissible. Speaker 4, a physician and bioethicist concerned with ethics and care at the end of life, will discuss conceptual issues regarding suffering, power, authority, and the patient-physician relationship, arguing that the legalization of pMAID is unwise. Speakers will conclude the session by outlining areas of agreement among all speakers and showing as precisely as possible where and why disagreement occurs.
Presenter
Faculty and Staff | Hecht-Levi Fellows and Students

The Ethics and Governance of Digital Contact Tracing Technologies for Pandemic Response
4:45 – 6:00 PM EST – ZOOM ROOM 15
PANEL PRESENTATION (LIVE) – PANDEMIC, POLICING, AND DIGITAL DATA
Presenter: Amelia Hood
Co-authors: Jeffrey Kahn, Joseph Ali, Anne Barnhill, Anita Cicero, Katelyn Esmonde, Brian Hutler, Alan Regenberg, Crystal Watson, Matthew Watson
As public health professionals around the world work tirelessly to respond to the COVID-19 pandemic, it is clear that traditional methods of contact tracing need to be augmented in order to help address a public health crisis of unprecedented scope. Rapidly developing digital technologies may aid public health surveillance and containment strategies for this pandemic and become part of the larger toolbox for future infectious outbreak prevention and control. The Johns Hopkins Project on Ethics and Governance of Digital Contact Tracing Technologies carried out an in-depth analysis of the technology and the issues it raises. Bringing together perspectives from bioethics, health security, public health, technology development, public policy, and law, we present clear and detailed guidelines to help manage the responsible creation, implementation, and application of digital contact tracing – prioritizing alignment of technology with public health needs and public values, building choice into design architecture, addressing equity considerations, and capturing real-world results and impacts to allow for continuous learning.

The Ethics and Governance of Digital Contact Tracing Technologies for Pandemic Response
4:45 – 6:00 PM EST – ZOOM ROOM 15
PANEL PRESENTATION (LIVE) – PANDEMIC, POLICING, AND DIGITAL DATA
Presenter: Amelia Hood
Co-authors: Jeffrey Kahn, Joseph Ali, Anne Barnhill, Anita Cicero, Katelyn Esmonde, Brian Hutler, Alan Regenberg, Crystal Watson, Matthew Watson
As public health professionals around the world work tirelessly to respond to the COVID-19 pandemic, it is clear that traditional methods of contact tracing need to be augmented in order to help address a public health crisis of unprecedented scope. Rapidly developing digital technologies may aid public health surveillance and containment strategies for this pandemic and become part of the larger toolbox for future infectious outbreak prevention and control. The Johns Hopkins Project on Ethics and Governance of Digital Contact Tracing Technologies carried out an in-depth analysis of the technology and the issues it raises. Bringing together perspectives from bioethics, health security, public health, technology development, public policy, and law, we present clear and detailed guidelines to help manage the responsible creation, implementation, and application of digital contact tracing – prioritizing alignment of technology with public health needs and public values, building choice into design architecture, addressing equity considerations, and capturing real-world results and impacts to allow for continuous learning.

Linguistic Bias in Language Used by Physicians in Medical Records of African American and White Patients: A Case of Testimonial Injustice?
12:30 – 1:00 PM – ZOOM ROOM 4
PAPER PRESENTATION Q&A (LIVE) – RACE, BIAS, ACCESS, AND DISPARITIES
Presenter: Mary Catherine Beach
Co-author: Marielle Gross
Background. African Americans and women report having medical concerns ignored or downplayed by health professionals. We explored racial/ethnic and gender differences in language suggesting disbelief of patients within medical records.
Methods. We previously identified 3 relevant linguistic features: (1) quotes (e.g.“reaction”); (2) negative words (e.g. claims, adamant); and (3) clausal complements, a sentence construction where the source of information is conveyed by providing key information as a clause (e.g. pt reports that headache started yesterday vs. the headache started yesterday). Although clausal complements are common in medical records and do not cast explicit doubt, we hypothesized that the overall number of sentences with that format may reflect greater doubt. We used natural language processing to identify features in ambulatory internal medicine notes written in 2017, and tested race/gender differences using mixed-effects Poisson regression accounting for clustering within patients and providers.
Results. Our sample included 7,986 notes written by 164 clinicians about 1890 patients (801 Black women, 630 Black men, 254 white women, and 205 white men). Notes written about Black patients had a greater number of quotes (p=0.039), negative language (p=0.006) and clausal complements (p<0.001). Analyses by gender were mixed: notes about female vs. male patients did not differ in negative language but had a greater number of quotes (p=0.002) and fewer clausal complements (p<0.001). There were no significant interactions between race and gender.
Discussion. African American patients may be subject to systematic bias in physicians’ perceptions of their credibility. Further studies are needed to understand these phenomena.
Lazy, Selfish Sluts: Shaming the ‘Bad Mother’ in Prenatal Records
SATURDAY, 2:15 – 2:45 PM EST – ZOOM ROOM 9
PAPER PRESENTATION Q&A (LIVE) – PATIENT TRUST AND PATIENT SHAMING
Presenter: Diana Mendoza-Cervantes
Co-authors: Marielle Gross, Mary Catherine Beach
Clinicians’ language within medical records may reflect biases and transmit stigma to other providers. Here, we examine the language in the medical records of 100 randomly-selected women admitted in 2017 for labor and delivery reflecting judgements against pregnant women qua mothers and the emergent theme of inappropriate presumption, or accusation, of maternal-fetal conflict. We highlight three subcategories implying woman are harming their fetus by failing to act (neglectfulness), acting in self-interest, and “moral contamination.” We also investigate potential racial and socioeconomic disparities in judgmental language. First, comments imply neglectfulness by emphasizing nonadherence to standard-of-care despite awareness of recommendations (“Has yet to complete 28 week labs,” “knows she should be taking iron, but not taking iron,” or “advanced maternal age, but declines genetic screening.” Second, comments depict patients’ decisions as selfish and threatening fetal well-being. For example, “aware of increased risk of maternal or neonatal morbidity and mortality, but still desires trial of labor,” or “hasn’t paid attention to fetal movements because of painful contractions.” Third, language stressing poor self-care, especially related to substance use or risky sex, may appear out of context or out of proportion to clinical significance, implying morally dubious character unbefitting of the modesty, chasteness or responsibleness of the “good mother” (e.g., history of chlamydia appearing five times in a History & Physical note). This category evokes concern that the mother imposes harm via “contamination” of the “innocent” fetus. We consider the impact physicians’ judgement of women as “bad mothers” may have on health outcomes.
Access the pre-recorded paper presentation here.

Management of Collateral Findings in PCTs
SATURDAY, 4:15 – 5:30 PM EST – ZOOM ROOM 11
PANEL PRESENTATION (LIVE)
Moderator: Stephanie Morain
Presenters: Stephanie Morain, Debra Mathews, Juli Bollinger
Unanticipated findings pose practical, ethical, and legal challenges for researchers, clinicians, institutions, and patients/research participants. While considerable attention has focused on unanticipated findings in clinical care and explanatory research, these issues have not been broadly considered in the context of pragmatic clinical trials (PCTs). Yet managing these “collateral findings” (CFs) in the context of PCTs is challenging, as many PCTs are done without patient consent and/or awareness of the research being done. Furthermore, the substantial scale of PCTs means that CF management may involve considerable resources and efforts for clinicians and health systems. As NIH invests substantial resources to expanding the use of PCTs, there is a critical need for guidance on PCT-CF management. In this session, we will introduce a conceptual overview and background regarding PCT-CFs, and outline why existing guidance has limited applicability for the PCT context. We will then present recent empirical data from three projects that elicited the perspectives of relevant stakeholders regarding their knowledge, attitudes, beliefs and expectations regarding PCT-CFs. First, we will describe the results of qualitative interviews with 60 key stakeholders involved in the conduct, design, and oversight of PCTs, including researchers, clinicians, IRB members, and health system leaders. Second, we will describe the results of 9 focus groups with patients, conducted at 3 different sites to reflect geographic diversity. Third, we will present the results of a national survey of the public. We will then engage the audience in a moderated discussion regarding lessons for the ethical management of PCT-CFs.

Social Justice and Bioethics through the Lens of the Story of Henrietta Lacks
1:15 – 2:30 PM EST – ZOOM ROOM 1
PLENARY
Moderator: Jeffrey Kahn
Presenters: Ruth Faden, Jeri Lacks, Victor Vines, Patricia King
Social justice and bioethics have never been more important than in our current times. This plenary session will feature a moderated panel discussion examining social justice and bioethics through the lens of issues and challenges raised by the story of Henrietta Lacks and the HeLa cell line derived from her cells. Topics include structural injustice; the perspective of members of the Lacks family; a retrospective on law, social justice, and bioethics from the release of the Belmont Report in 1979 to the present; and a discussion of how architecture and design can represent legacy and naming. An audience Q&A will follow the panel presentations.
Advancing Ethical HIV Research in Pregnancy: Guidance from the PHASES Working Group
2:15 – 3:30 PM EST – ZOOM ROOM 13
PANEL PRESENTATION (LIVE)
Moderator: Kristen Sullivan
Presenters: Kristen Sullivan, Annie Lyerly, Ruth Faden, Maggie Little
Numerous barriers to the inclusion of pregnant women in biomedical research have resulted in profound and consequential evidence gaps about how to safely and effectively treat and prevent HIV and co-infections during pregnancy. This puts women and the children they bear in harm’s way. Without timely pregnancy-specific data on safety, drugs may be used that carry unacceptable risks to the fetus or pregnant woman. Additionally, in the absence of pregnancy-specific data on how drugs interact with the distinctive physiology of pregnancy, pregnant women may be given doses that are too low or too high. Perhaps most profoundly, delays in gathering evidence can mean a delay in pregnant women’s access to next-generation drugs — and the important benefits they may entail over older drug options. In order to address this pressing need and advance timely, responsible research with pregnant women, the Pregnancy and HIV/AIDS Seeking Equitable Study (PHASES) Working Group – an international, interdisciplinary, and inter-sectoral group of 26 individuals from across the HIV research and advocacy community – developed concrete ethics guidance, informed by extensive stakeholder engagement. This panel presentation will: 1) orient participants to the harms created by the evidence gap for pregnant women in HIV/co-infection prevention and treatment; 2) describe PHASES’ empirical research that informed the Guidance; 3) articulate the ethical foundations of the Guidance, and 4) share the 12 recommendations in the Guidance, directed to multiple stakeholders, which aim to secure better, earlier, and more systematic evidence on safely and effectively treating and preventing HIV and co-infections during pregnancy.

Should Genomics Play a Role in Clinical Management of High Consequence Infections?
2:15 – 2:45 PM EST – ZOOM ROOM 4
PAPER PRESENTATION Q&A (LIVE) – CLINICAL ETHICS AND EMERGING BIOTECHNOLOGIES
Presenter: Gail Geller
Co-authors: Jennifer Gerber, Brian Garibaldi
We are currently in the midst of a coronavirus epidemic. High-level isolation units (HLIUs) (funded by the U.S. Office of the Assistant Secretary for Preparedness and Response after the 2014-2016 Ebola outbreak) are gearing up to care for patients with COVID-19. Evidence from other high consequence infectious diseases (HCIDs) including viral hemorrhagic fevers, SARS, and novel influenza viruses, suggests that host genomics influences susceptibility to such infections, vaccine and treatment response, and morbidity and mortality. Should HLIUs incorporate genomics in patient care? We surveyed HLIU staff to identify the ethical, legal and social implications (ELSI) of using genomic technologies in this environment. 112 staff representing all 10 HLIUs responded. Over 85% agreed with the use of genomic testing to identify patients at risk for poor outcomes from HCIDs, including treatment non-response, morbidity and mortality. However, 30% strongly disagreed with using genomic information to make treatment decisions. 75% felt that it would be unethical to withhold treatment from patients with a high likelihood of mortality, and more than half felt admission to a unit should not be impacted by genomics. 90% would support confidential employer-based testing of healthcare workers to understand individual staff risk, but 84% felt this information should not inform work assignments. 89% of respondents felt they would benefit from additional education on genomics. Our results highlight ELSI concerns about genomic testing in HLIUs, and can inform future research, workforce privacy/discrimination policies and patient treatment allocation decisions regarding the role of genomic testing of patients and staff in HLIUs.
Access the pre-recorded paper presentation here.
Antigone in Ferguson
6:00 – 8:30 PM EST – ZOOM ROOM 1
PERFORMANCE AND EXHIBITION (LIVE)
Panel: Jeffrey Kahn, Gail Geller
Antigone in Ferguson is a groundbreaking project that fuses dramatic readings by acclaimed actors of Sophocles’ Antigone with live choral music performed by a diverse choir, from St. Louis, Missouri and New York City culminating in powerful, healing discussions about racialized violence, police brutality, systemic oppression, gender-based violence, health inequality, and social justice. The project was conceived in the wake of Michael Brown’s death in 2014, through a collaboration between Theater of War Productions and community members from Ferguson, MO, and premiered at Normandy High School, Michael Brown’s alma mater, in September of 2016, and has since toured the country and the world. In light of the uprising and protests catalyzed by the killings of Ahmaud Arbery, Breonna Taylor, George Floyd, Tony McDade, Dion Johnson, and many others; and the disproportionate impact of the COVID-19 pandemic on black and brown communities, Antigone in Ferguson aims to generate dialogue, consciousness, compassion, outrage, understanding, and positive action at this critical moment. This special presentation of Antigone in Ferguson on Zoom will foreground the perspectives of people in Baltimore, Maryland whose lives have been impacted by police brutality, community violence, and the COVID-19 pandemic. Co-presented by Theater of War Productions, the Johns Hopkins Berman Institute of Bioethics, and the Johns Hopkins Program in Arts, Humanities & Health.
Watch a preview and register online.
Linguistic Bias in Language Used by Physicians in Medical Records of African American and White Patients: A Case of Testimonial Injustice?
12:30 – 1:00 PM – ZOOM ROOM 4
PAPER PRESENTATION Q&A (LIVE) – RACE, BIAS, ACCESS, AND DISPARITIES
Presenter: Mary Catherine Beach
Co-author: Marielle Gross
Background. African Americans and women report having medical concerns ignored or downplayed by health professionals. We explored racial/ethnic and gender differences in language suggesting disbelief of patients within medical records.
Methods. We previously identified 3 relevant linguistic features: (1) quotes (e.g.“reaction”); (2) negative words (e.g. claims, adamant); and (3) clausal complements, a sentence construction where the source of information is conveyed by providing key information as a clause (e.g. pt reports that headache started yesterday vs. the headache started yesterday). Although clausal complements are common in medical records and do not cast explicit doubt, we hypothesized that the overall number of sentences with that format may reflect greater doubt. We used natural language processing to identify features in ambulatory internal medicine notes written in 2017, and tested race/gender differences using mixed-effects Poisson regression accounting for clustering within patients and providers.
Results. Our sample included 7,986 notes written by 164 clinicians about 1890 patients (801 Black women, 630 Black men, 254 white women, and 205 white men). Notes written about Black patients had a greater number of quotes (p=0.039), negative language (p=0.006) and clausal complements (p<0.001). Analyses by gender were mixed: notes about female vs. male patients did not differ in negative language but had a greater number of quotes (p=0.002) and fewer clausal complements (p<0.001). There were no significant interactions between race and gender.
Discussion. African American patients may be subject to systematic bias in physicians’ perceptions of their credibility. Further studies are needed to understand these phenomena.
“Safe Harbor” for Deidentified Data as a Barrier to Ethical Learning Health Systems
4:15 – 4:45 PM EST – ZOOM ROOM 8
PAPER PRESENTATION Q&A (LIVE) – PATIENT DATA AND PATIENT PRIVACY
Presenter: Marielle Gross
To promote learning while minimizing privacy risks in the U.S., deidentified health data is neither restricted by HIPAA nor governed by Common Rule human subjects’ protections (AKA “safe harbor”). Meanwhile, the ethical framework for learning health systems (LHS) proposes both that clinical care and research no longer be treated as ethically distinct, and that patients are obligated to contribute to learning, primarily by allowing research on health data generated during clinical care. We argue that patient-centered outcomes research, which involves questions and outcomes “meaningful and important to patients and caregivers,” under current “safe harbor” for deidentified data may undermine distributive justice, respect for persons and beneficence. First, deidentification intentionally eliminates accountability to patients whose data are studied, making research easier, but also precluding patients from direct clinical benefits or recognition. This contradicts just distribution of research benefits and burdens, especially when findings are timely or personally significant, exacerbated by background conditions in which access to healthcare is not universal. Further, lack of human subjects’ protections may functionally dehumanize deidentified patients by allowing commodification or monetization of inherently intimate data—data often obtained via invasive examinations performed under auspices of advancing individuals’ health interests—without consent. Finally, deidentification’s beneficent intentions are challenged by artificial intelligence coupled with increasing clinical, genomic and biological data, and by systematically emphasizing siloed, incomplete datasets. Ultimately, deidentification may impede flourishing of a just LHS by maintaining the distinction between patient and research subject, neglecting duties of care to both, and preventing seamless treatment, learning and feedback.
Contemporary Ethical Controversy in Fetal Therapy: Innovation, Research, Access, and Justice
5:00 – 6:15 PM EST – ZOOM ROOM 16
PANEL PRESENTATION (LIVE)
Moderator: Naomi Laventhal
Presenter: John Lantos, Armand Antommaria, Marielle Gross
Maternal-fetal care centers offer an increasingly broad range of fetal therapies to improve survival and quality-of-life for infants with congenital anomalies. Most interventions are not well-studied, raising ethical issues for clinician-scientists, patients, policy makers, and research institutions. Should these interventions be classified as experimental and only offered under IRB approved protocols? How should women be counseled regarding ultrasound findings for which fetal therapies may offer benefit when they are neither widely available nor standard-of-care? To what lengths should a “good mother” go for her baby? How should fetal interventions be studied in the context of regulatory complexity, competing stakeholder interests, diagnostic and prognostic uncertainties, and small patient populations? We explore some of the pressing and unresolved ethical questions about fetal intervention research.The moderator, a neonatologist, bioethicist, and expert in antenatal consultation, will introduce the salient issues using an illustrative case. A pediatrician and expert in neonatal and research ethics will examine in the tension between innovation and research, with attention to how families and clinicians make decisions to pursue these interventions. Another pediatrician bioethicist will explore when innovative therapy should be subject to systematic evaluation and what types of experimental designs and eligibility criteria are appropriate with attention to equitable access to potential treatments. An OB/GYN bioethicist will examine ethical, legal, and social dimensions of care for pregnant women in the face of emerging, paradigm-shifting, surgical and genetic technologies, disparities, legal constraints to reproductive choice, and sociological context of idealization/judgement of pregnant women qua mothers.
Behind the Curtain: An Ethical Analysis of Menstruation Tracking App Data
FRIDAY, 12:00 – 12:30 PM EST – ZOOM ROOM 1
PAPER PRESENTATION Q&A (LIVE) – MOBILE HEALTH APPLICATIONS, HEALTH INFORMATION SYSTEMS, AND HEALTHCARE METRICS
Presenter: Amelia Hood
Co-authors: Marielle Gross, Bethany Corbin, Margot Kelly-Hedrick
The recent explosion of digital health applications includes emergence and growing popularity of smartphone apps for women’s health, most commonly menstruation tracking apps. Meanwhile, monetization of women’s sensitive health data has led to public outcry and ethical concerns. Here, we explore menstruation apps as a case study of the creation of health data outside of traditional healthcare contexts from a medico-legal and justice perspective. Menstrual apps normalize a new frontier for ubiquitous data surveillance, gathering health-relevant data outside of the traditional healthcare contexts where it may not be afforded appropriate legal or ethical protection. App companies provide “free” services and then generate profits from user data by selling targeted advertisements for health-related products and healthcare services. Many health apps raise concerns regarding their effectiveness for promoting health— The services offered by the app, namely ovulation and fertility prediction, have been found to be inaccurate, sometimes with life-altering consequences. For example, the Natural Cycles app, the first app to have FDA clearance for its contraceptive algorithm, enticed many women to utilize it as their sole birth control method, but notoriously failing, with many unintended pregnancies resulting. We identify and discuss two primary concerns: 1. Menstrual apps impersonate healthcare without appropriate expertise, oversight or fiduciary duties. 2. Menstrual apps, largely funded and designed by men, commodify women’s bodies, treating their reproductive systems as a source of profit, ultimately exploiting their asymmetric power by systematically taking more value (women’s data and role as consumers of advertisements) than they provide (app services).
Lazy, Selfish Sluts: Shaming the ‘Bad Mother’ in Prenatal Records
SATURDAY, 2:15 – 2:45 PM EST – ZOOM ROOM 9
PAPER PRESENTATION Q&A (LIVE) – PATIENT TRUST AND PATIENT SHAMING
Presenter: Diana Mendoza-Cervantes
Co-authors: Marielle Gross, Mary Catherine Beach
Clinicians’ language within medical records may reflect biases and transmit stigma to other providers. Here, we examine the language in the medical records of 100 randomly-selected women admitted in 2017 for labor and delivery reflecting judgements against pregnant women qua mothers and the emergent theme of inappropriate presumption, or accusation, of maternal-fetal conflict. We highlight three subcategories implying woman are harming their fetus by failing to act (neglectfulness), acting in self-interest, and “moral contamination.” We also investigate potential racial and socioeconomic disparities in judgmental language. First, comments imply neglectfulness by emphasizing nonadherence to standard-of-care despite awareness of recommendations (“Has yet to complete 28 week labs,” “knows she should be taking iron, but not taking iron,” or “advanced maternal age, but declines genetic screening.” Second, comments depict patients’ decisions as selfish and threatening fetal well-being. For example, “aware of increased risk of maternal or neonatal morbidity and mortality, but still desires trial of labor,” or “hasn’t paid attention to fetal movements because of painful contractions.” Third, language stressing poor self-care, especially related to substance use or risky sex, may appear out of context or out of proportion to clinical significance, implying morally dubious character unbefitting of the modesty, chasteness or responsibleness of the “good mother” (e.g., history of chlamydia appearing five times in a History & Physical note). This category evokes concern that the mother imposes harm via “contamination” of the “innocent” fetus. We consider the impact physicians’ judgement of women as “bad mothers” may have on health outcomes.
Access the pre-recorded paper presentation here.

Behind the Curtain: An Ethical Analysis of Menstruation Tracking App Data
FRIDAY, 12:00 – 12:30 PM EST – ZOOM ROOM 1
PAPER PRESENTATION Q&A (LIVE) – MOBILE HEALTH APPLICATIONS, HEALTH INFORMATION SYSTEMS, AND HEALTHCARE METRICS
Presenter: Amelia Hood
Co-authors: Marielle Gross, Bethany Corbin, Margot Kelly-Hedrick
The recent explosion of digital health applications includes emergence and growing popularity of smartphone apps for women’s health, most commonly menstruation tracking apps. Meanwhile, monetization of women’s sensitive health data has led to public outcry and ethical concerns. Here, we explore menstruation apps as a case study of the creation of health data outside of traditional healthcare contexts from a medico-legal and justice perspective. Menstrual apps normalize a new frontier for ubiquitous data surveillance, gathering health-relevant data outside of the traditional healthcare contexts where it may not be afforded appropriate legal or ethical protection. App companies provide “free” services and then generate profits from user data by selling targeted advertisements for health-related products and healthcare services. Many health apps raise concerns regarding their effectiveness for promoting health— The services offered by the app, namely ovulation and fertility prediction, have been found to be inaccurate, sometimes with life-altering consequences. For example, the Natural Cycles app, the first app to have FDA clearance for its contraceptive algorithm, enticed many women to utilize it as their sole birth control method, but notoriously failing, with many unintended pregnancies resulting. We identify and discuss two primary concerns: 1. Menstrual apps impersonate healthcare without appropriate expertise, oversight or fiduciary duties. 2. Menstrual apps, largely funded and designed by men, commodify women’s bodies, treating their reproductive systems as a source of profit, ultimately exploiting their asymmetric power by systematically taking more value (women’s data and role as consumers of advertisements) than they provide (app services).
Access the pre-recorded paper presentation here.
The Ethics and Governance of Digital Contact Tracing Technologies for Pandemic Response
FRIDAY, 4:45 – 6:00 PM EST – ZOOM ROOM 15
PANEL PRESENTATION (LIVE) – PANDEMIC, POLICING, AND DIGITAL DATA
Presenter: Amelia Hood
Co-authors: Jeffrey Kahn, Joseph Ali, Anne Barnhill, Anita Cicero, Katelyn Esmonde, Brian Hutler, Alan Regenberg, Crystal Watson, Matthew Watson
As public health professionals around the world work tirelessly to respond to the COVID-19 pandemic, it is clear that traditional methods of contact tracing need to be augmented in order to help address a public health crisis of unprecedented scope. Rapidly developing digital technologies may aid public health surveillance and containment strategies for this pandemic and become part of the larger toolbox for future infectious outbreak prevention and control. The Johns Hopkins Project on Ethics and Governance of Digital Contact Tracing Technologies carried out an in-depth analysis of the technology and the issues it raises. Bringing together perspectives from bioethics, health security, public health, technology development, public policy, and law, we present clear and detailed guidelines to help manage the responsible creation, implementation, and application of digital contact tracing – prioritizing alignment of technology with public health needs and public values, building choice into design architecture, addressing equity considerations, and capturing real-world results and impacts to allow for continuous learning.

Social Justice and Bioethics through the Lens of the Story of Henrietta Lacks
THURSDAY, 1:15 – 2:30 PM EST – ZOOM ROOM 1
PLENARY
Moderator: Jeffrey Kahn
Presenters: Ruth Faden, Jeri Lacks, Victor Vines, Patricia King
Social justice and bioethics have never been more important than in our current times. This plenary session will feature a moderated panel discussion examining social justice and bioethics through the lens of issues and challenges raised by the story of Henrietta Lacks and the HeLa cell line derived from her cells. Topics include structural injustice; the perspective of members of the Lacks family; a retrospective on law, social justice, and bioethics from the release of the Belmont Report in 1979 to the present; and a discussion of how architecture and design can represent legacy and naming. An audience Q&A will follow the panel presentations.
The Ethics and Governance of Digital Contact Tracing Technologies for Pandemic Response
FRIDAY, 4:45 – 6:00 PM EST – ZOOM ROOM 15
PANEL PRESENTATION (LIVE) – PANDEMIC, POLICING, AND DIGITAL DATA
Presenter: Amelia Hood
Co-authors: Jeffrey Kahn, Joseph Ali, Anne Barnhill, Anita Cicero, Katelyn Esmonde, Brian Hutler, Alan Regenberg, Crystal Watson, Matthew Watson
As public health professionals around the world work tirelessly to respond to the COVID-19 pandemic, it is clear that traditional methods of contact tracing need to be augmented in order to help address a public health crisis of unprecedented scope. Rapidly developing digital technologies may aid public health surveillance and containment strategies for this pandemic and become part of the larger toolbox for future infectious outbreak prevention and control. The Johns Hopkins Project on Ethics and Governance of Digital Contact Tracing Technologies carried out an in-depth analysis of the technology and the issues it raises. Bringing together perspectives from bioethics, health security, public health, technology development, public policy, and law, we present clear and detailed guidelines to help manage the responsible creation, implementation, and application of digital contact tracing – prioritizing alignment of technology with public health needs and public values, building choice into design architecture, addressing equity considerations, and capturing real-world results and impacts to allow for continuous learning.
Antigone in Ferguson
SATURDAY, 6:00 – 8:30 PM EST – ZOOM ROOM 1
PERFORMANCE AND EXHIBITION (LIVE)
Panel: Jeffrey Kahn, Gail Geller
Antigone in Ferguson is a groundbreaking project that fuses dramatic readings by acclaimed actors of Sophocles’ Antigone with live choral music performed by a diverse choir, from St. Louis, Missouri and New York City culminating in powerful, healing discussions about racialized violence, police brutality, systemic oppression, gender-based violence, health inequality, and social justice. The project was conceived in the wake of Michael Brown’s death in 2014, through a collaboration between Theater of War Productions and community members from Ferguson, MO, and premiered at Normandy High School, Michael Brown’s alma mater, in September of 2016, and has since toured the country and the world. In light of the uprising and protests catalyzed by the killings of Ahmaud Arbery, Breonna Taylor, George Floyd, Tony McDade, Dion Johnson, and many others; and the disproportionate impact of the COVID-19 pandemic on black and brown communities, Antigone in Ferguson aims to generate dialogue, consciousness, compassion, outrage, understanding, and positive action at this critical moment. This special presentation of Antigone in Ferguson on Zoom will foreground the perspectives of people in Baltimore, Maryland whose lives have been impacted by police brutality, community violence, and the COVID-19 pandemic. Co-presented by Theater of War Productions, the Johns Hopkins Berman Institute of Bioethics, and the Johns Hopkins Program in Arts, Humanities & Health.
Watch a preview and register online.
A Plague of Darkness: Justice, Ethics and COVID-19
SUNDAY, 11:30 AM – 12:45 PM EST – ZOOM ROOM 9
PANEL PRESENTATION (LIVE)
Moderator: Laurie Zoloth
Presenters: Laurie Zoloth, Jeffrey Kahn, Jeffrey Bishop, Gayman Bennett
COVID-19 emerged from the center of industrialized China in the late winter of 2020 from under the cover of censorship and consistent public denial. While Americans watched, China finally mounted a massive public health trial, unprecedented in the modern era, of a cordon sanitaire around nearly half of the county. It did not work to contain the epidemic, which as of the submission deadline, has just begun community spread in Europe, the Middle East, and the United States. The epidemic pulls questions of justice that bioethics has been debating for decade into the center of the epidemic. The closing of hospitals has left only 62,000 ICU beds with ventilators available for 300 million people; the bulk of citizens do not have sick days, coverage for tests; the ability to stay home from hourly jobs; to stock up on products in large amounts, or have the spare bathroom that CDC in its imaginary, recommends. Our culture is based on the capacity of the poorest to care for the elderly, to cook and serve, yet it is these people who are most vulnerable. The epidemic also displays the tension between libertarian systems of justice and communitarian ones, between social isolation and human gathering, such as religious or political public spaces. We will explore the profound social dynamics of the epidemics from four different perspectives within the claim that it represents the most important challenge to bioethics and humanities in decades.

Engaging Policymakers on Bioethics Issues
WEDNESDAY, 11:30 AM – 2:30 PM EST – ZOOM ROOM 3
PRE-CONFERENCE WORKSHOP (LIVE)
Presenters: Aaron Levine, Debra Mathews, Beth Roxland, Emily Cloyd
This virtual workshop, led by an expert in science communication from the American Association of the Advancement of Science, provides best practices and actionable tools to allow participants to begin strategically engaging with policymakers and support the use of bioethics expertise in the policymaking process. The session starts with an overview of the science and health policy landscape and the role of scientists and bioethicists in the policy process. The workshop introduces best practices for working with policymakers at a local, state or national level, including strategies for accessing and engaging with policymakers. The workshop includes facilitator presentations and individual and small group exercises using virtual breakout groups. Each participant will identify an individual communication goal and develop short messages that will resonate with specific policy audiences. In addition, participants will rehearse an in-person meeting with a policymaker, such as a member of Congress or her/his staff, gaining practice with this important form of policy engagement. Key best practices will be illustrated with examples from ASBH members experienced in engaging with policymakers. Exemplars will include Debra Mathews, who will share lessons learned from her experiences testifying before legislative bodies at the state and federal level and serving as a staff member on the Presidential Commission for the Study of Bioethical Issues, and Beth Roxland, who will draw on her experience as Executive Director of the New York State Task Force on Life and the Law, including her role helping enact the Task Force’s Brain Death Report into state regulations, and as policy analyst and advisor.
Building Diversity in ELSI: Reflections from Training Program Directors
10:45 – 11:45 AM EST – ZOOM ROOM 11
ELSI AFFINITY GROUP MEETING
Moderator: Aaron Goldberg
Panelists: Debra Mathews, James Tabery
Management of Collateral Findings in PCTs
SATURDAY, 4:15 – 5:30 PM EST – ZOOM ROOM 11
PANEL PRESENTATION (LIVE)
Moderator: Stephanie Morain
Presenters: Stephanie Morain, Debra Mathews, Juli Bollinger
Unanticipated findings pose practical, ethical, and legal challenges for researchers, clinicians, institutions, and patients/research participants. While considerable attention has focused on unanticipated findings in clinical care and explanatory research, these issues have not been broadly considered in the context of pragmatic clinical trials (PCTs). Yet managing these “collateral findings” (CFs) in the context of PCTs is challenging, as many PCTs are done without patient consent and/or awareness of the research being done. Furthermore, the substantial scale of PCTs means that CF management may involve considerable resources and efforts for clinicians and health systems. As NIH invests substantial resources to expanding the use of PCTs, there is a critical need for guidance on PCT-CF management. In this session, we will introduce a conceptual overview and background regarding PCT-CFs, and outline why existing guidance has limited applicability for the PCT context. We will then present recent empirical data from three projects that elicited the perspectives of relevant stakeholders regarding their knowledge, attitudes, beliefs and expectations regarding PCT-CFs. First, we will describe the results of qualitative interviews with 60 key stakeholders involved in the conduct, design, and oversight of PCTs, including researchers, clinicians, IRB members, and health system leaders. Second, we will describe the results of 9 focus groups with patients, conducted at 3 different sites to reflect geographic diversity. Third, we will present the results of a national survey of the public. We will then engage the audience in a moderated discussion regarding lessons for the ethical management of PCT-CFs.

Taking Justice Seriously in Health Research and Policy in Low-Resource Settings
SATURDAY, 4:15 – 5:30 PM EST – ZOOM ROOM 8
PANEL PRESENTATION (LIVE)
Moderator: Maria Merritt
Presenters: Maureen Kelley, Danielle Wenner, Maria Merritt
Three presenters will speak for 15 minutes each. Then the moderator will facilitate audience-presenter dialogue for 30 minutes: approximately 10 minutes for each of three learning objectives.1. Justice-based obligations in health research priority-setting. How far do health research priority-setting obligations extend? The presenter will argue that (a) research funders from high-income countries have priority-setting obligations that extend beyond national borders; and (b) such funders have greater obligations to prioritize research questions that address disease burdens in low-resource settings, while epistemic communities in low-resource settings should prioritize local needs. This approach is suggested by understanding how international research institutions condition global citizens’ life chances.2. How do health researchers address structural injustice? To what extent do researchers engage structural sources of vulnerability in low-resource settings, and what are the implications for research ethics practice? The presenter will describe qualitative findings from an international empirical research ethics study involving six “embedded ethics” case studies in low-resource settings where bioethics teams linked within ongoing biomedical research. This talk will discuss cross-site findings illustrating how researchers navigate participant and community needs arising from deeper social, economic, and political injustices. 3. Representing social justice in health-related policy decisions. How can social justice be represented in comparing health policy options under resource constraints? The presenter will describe a sustained example of using philosophical social justice theory and qualitative research to develop user-friendly analytic tools that supplement traditional economic evaluation, with special attention to enabling the use of local data and local capacity at low cost.

Is This Child Dead? Controversies Regarding the Neurological Criteria for Death
SATURDAY, 4:15 – 5:30 PM EST – ZOOM ROOM 3
PANEL PRESENTATION (LIVE)
Moderator: Lainie Ross
Presenters: Lainie Ross, Armand Antommaria, Margaret Moon
One hundred years ago, patients were simply declared dead when their respiration and circulation ceased. However, in 1968, an Ad Hoc Committee of the Harvard Medical School published a report that provided criteria for determination of death by neurological criteria. The Uniform Determination of Death Act (UDDA), proposed in 1981, added the neurological criteria to the cardio-respiratory criteria and states that “A determination of death must be made in accordance with accepted medical standards.” Professional association have subsequently approved guidelines for determining when individuals fulfill the neurological criteria including apnea testing. While many states have adopted the UDDA’s language, several provide forms of accommodation for individuals who reject the neurological criteria. This regime has come under criticism by clinicians and philosophers who argue that these criteria do not accurately determine when individuals have died and has been challenged by families who wish to continue treatment for their loved ones. In this session, three pediatricians will discuss different aspects of the brain death debate and its implications for children. The first speaker, a pediatrician-philosopher, will argue that informed consent for apnea testing is only needed if families are able to choose the criteria by which their loved ones are declared dead. A second speaker, a pediatrician-religious ethicist, will discuss accommodating differing views of death including whether public insurance should cover continued treatment for individuals who fulfill the neurological criteria. The third speaker, a pediatrician-clinical ethicist-hospital administrator, will discuss the practical challenges of applying well-reasoned ethics policies in complex, modern hospital systems.
Perspectives on Physician-Assisted Dying for Persons with Psychiatric Illnesses
SUNDAY, 1:45 – 3:00 PM EST – ZOOM ROOM 2
DEBATE (LIVE)
Moderator: Marie Nolan
Presenters: Brent Kious, Margaret ‘Peggy’ Battin, Scott Kim, Daniel Sulmasy
Physician-assisted dying, or medical aid-in-dying (MAID), is legal in ten US jurisdictions but only for persons with terminal illnesses (tMAID). In several European countries, euthanasia or assisted suicide, as it is called there, is permitted for persons without terminal illness but with unbearable suffering, including psychiatric illness (pMAID). pMAID remains controversial even in jurisdictions where it is permitted. Moderator Marie Nolan will present background information about MAID in the US and Europe. Speaker 1, a bioethicist and philosopher familiar with MAID in the Netherlands and with historical sources concerning the ethics of suicide, will question whether the suffering of persons with psychiatric illnesses can sometimes justify access to pMAID. Speaker 2, a psychiatrist and philosopher-bioethicist who has conducted research specifically regarding pMAID in the Netherlands, will argue that equality arguments to expand pMAID are flawed and that there are strong policy and clinical considerations against pMAID. Speaker 3, a psychiatrist and philosopher-bioethicist who examines the role of values in medicine, will focus on treatment-refractory psychiatric illness, arguing that it can involve suffering as serious as that in physical illness; hence pMAID is permissible. Speaker 4, a physician and bioethicist concerned with ethics and care at the end of life, will discuss conceptual issues regarding suffering, power, authority, and the patient-physician relationship, arguing that the legalization of pMAID is unwise. Speakers will conclude the session by outlining areas of agreement among all speakers and showing as precisely as possible where and why disagreement occurs.

The Ethics and Governance of Digital Contact Tracing Technologies for Pandemic Response
FRIDAY, 4:45 – 6:00 PM EST – ZOOM ROOM 15
PANEL PRESENTATION (LIVE) – PANDEMIC, POLICING, AND DIGITAL DATA
Presenter: Amelia Hood
Co-authors: Jeffrey Kahn, Joseph Ali, Anne Barnhill, Anita Cicero, Katelyn Esmonde, Brian Hutler, Alan Regenberg, Crystal Watson, Matthew Watson
As public health professionals around the world work tirelessly to respond to the COVID-19 pandemic, it is clear that traditional methods of contact tracing need to be augmented in order to help address a public health crisis of unprecedented scope. Rapidly developing digital technologies may aid public health surveillance and containment strategies for this pandemic and become part of the larger toolbox for future infectious outbreak prevention and control. The Johns Hopkins Project on Ethics and Governance of Digital Contact Tracing Technologies carried out an in-depth analysis of the technology and the issues it raises. Bringing together perspectives from bioethics, health security, public health, technology development, public policy, and law, we present clear and detailed guidelines to help manage the responsible creation, implementation, and application of digital contact tracing – prioritizing alignment of technology with public health needs and public values, building choice into design architecture, addressing equity considerations, and capturing real-world results and impacts to allow for continuous learning.

Is Rarer Fairer? An Interactive Drama Explores Justice and Resources-Intensive Innovative Therapies for Rare Diseases
THURSDAY, 5:00 – 6:15 PM EST – ZOOM ROOM 10
PERFORMANCE AND EXHIBITION (LIVE)
Panel: Lynn Bush, Christine Mitchell, Karen Rothenberg, Robert Truog
This performance session premieres a multi-case drama, mock attendee advisory committee, and family video to actively engage the diverse ASBH community in conversations exploring notions of Justice and Fairness in the context of resource-intensive new interventions for rare genetic diseases. Both the play’s dialogue and two video excerpts illuminate the complex ethical landscape of translational medicine to critically examine implications, interests, perspectives, and tensions between individuals, families, communities, and our pluralistic society-at-large. Questions abound, such as who should pay the exorbitant costs for the development and administration of innovative therapies, how does an institution decide the inclusion criteria for access to the therapy, and should it make a difference whether the resource allocation may only benefit a small number even within the rare disease community? The twenty-minute play sets the stage to enhance reflective ethical consideration of justice issues and foster lively inter-professional discourse. After the performance, the multidisciplinary bioethicist-presenters (PhD; RN; JD; MD) and nation-wide troupe of distinguished bioethicist-actors, share insights based on their character’s role and own experiences. Broad audience discussion is elicited; participants are also encouraged to provide feedback regarding this narrative-genomic pedagogy as an approach for ethics education as well as interprofessional and community engagement. Attendees are then engaged in a mock Ethics Advisory Committee tasked with voting on select decision-making processes to guide policy for this fictional institution. Lastly, we present and discuss two powerful excerpts from a video shared by a family who has a child with a rare genetic condition.

Informing Ethical Policy: A Justice-Based Approach to Drug Shortages
11:00 AM – 12:15 PM EST – ZOOM ROOM 8
PANEL PRESENTATION (LIVE)
Presenters: Andrew Shuman, Megan Petry, Douglas Throckmorton, Yoram Unguru
Drug shortages are a national tragedy that represent a public health crisis preventing patients from accessing lifesaving medications. They represent a symptom of our inherently damaged healthcare system in which pharmaceutical companies are perversely dis-incentivized to produce inexpensive generic, but critical drugs. The absence of policies incentivizing quality and increased manufacturing of essential medicines further constrains our broken healthcare marketplace. These consequences of our current governance structure will require legislative and regulatory action. The panel features two practicing oncology doctors, a pediatrician and a surgeon, who are clinical ethicists studying responses and solutions to drug shortages; they will use the recent vincristine shortage as a paradigmatic example, review how we got here, and summarize an ethical framework to potential solutions. A senior administrator of the US Federal Drug Administration (FDA) who has been at the helm of federal responses to drug shortages and helped release a long-anticipated report that contextualized the current state of drug shortages will provide the regulatory perspective. In addition, an investigative counsel from the United States Senate Homeland Security and Governmental Affairs Committee minority staff who produced a report, “A Price Too High: Cost, Supply, and Security Threats to Affordable Prescription Drugs,” will share tangible and actionable legislative solutions. This multidisciplinary group will then discuss how federal policy, regulatory, and legislative changes can be directly informed by a shared framework of responsibilities in a manner that recognizes real-world constraints, but also ensures that patients in need are able to fairly access essential medications.
Responding to Treatment Refusal, Respecting Pluralistic Values and Retaining the Child’s Right to Flourish
THURSDAY, 2:45 – 4:00 PM EST – ZOOM ROOM 2
PANEL PRESENTATION (LIVE)
Moderator: Yoram Unguru
Presenters: Yoram Unguru, Amy Caruso Brown, Erin Paquette
Guided by the best interest standard, parents are granted wide latitude in making decisions for their children. Children and/or parent(s) may disagree with their physician’s treatment recommendation. There are, however, limits to the decisions society will allow children and parents to make on behalf of their children. Refusal of life-saving treatment for a potentially curable childhood cancer provokes strong emotions for members of the healthcare team often resulting in frustration and at times, moral distress. It is up to individual physicians to decide whether to report treatment refusal (TR) and initiate medical neglect proceedings. Among childhood cancer providers, evidence suggests that a sizable minority of pediatric oncologists opt not to report. Moreover, many physicians are more willing to accept requests for TR when the prognosis is “poor” and the parent and older child have made a mutual decision. In deciding whether to report, it is less clear whether physicians rely upon a specific survival threshold and how they grapple with prognostic uncertainty or burden and length of treatment. Similarly, how does physician bias and/or discrimination influence reporting decisions, specifically, patient/family factors such as race, culture, educational level, personality, and reason for TR (religious objection, desire for complementary/alternative therapies)?Viewed through the lens of “justice and flourishing” we consider the role for pluralistic values/morals in cases of TR. To frame the discussion and highlight many of these themes, we will present an actual case of an adolescent with curable cancer who along with his parents refused recommended care.

The Ethics and Governance of Digital Contact Tracing Technologies for Pandemic Response
FRIDAY, 4:45 – 6:00 PM EST – ZOOM ROOM 15
PANEL PRESENTATION (LIVE) – PANDEMIC, POLICING, AND DIGITAL DATA
Presenter: Amelia Hood
Co-authors: Jeffrey Kahn, Joseph Ali, Anne Barnhill, Anita Cicero, Katelyn Esmonde, Brian Hutler, Alan Regenberg, Crystal Watson, Matthew Watson
As public health professionals around the world work tirelessly to respond to the COVID-19 pandemic, it is clear that traditional methods of contact tracing need to be augmented in order to help address a public health crisis of unprecedented scope. Rapidly developing digital technologies may aid public health surveillance and containment strategies for this pandemic and become part of the larger toolbox for future infectious outbreak prevention and control. The Johns Hopkins Project on Ethics and Governance of Digital Contact Tracing Technologies carried out an in-depth analysis of the technology and the issues it raises. Bringing together perspectives from bioethics, health security, public health, technology development, public policy, and law, we present clear and detailed guidelines to help manage the responsible creation, implementation, and application of digital contact tracing – prioritizing alignment of technology with public health needs and public values, building choice into design architecture, addressing equity considerations, and capturing real-world results and impacts to allow for continuous learning.

The Ethics and Governance of Digital Contact Tracing Technologies for Pandemic Response
FRIDAY, 4:45 – 6:00 PM EST – ZOOM ROOM 15
PANEL PRESENTATION (LIVE) – PANDEMIC, POLICING, AND DIGITAL DATA
Presenter: Amelia Hood
Co-authors: Jeffrey Kahn, Joseph Ali, Anne Barnhill, Anita Cicero, Katelyn Esmonde, Brian Hutler, Alan Regenberg, Crystal Watson, Matthew Watson
As public health professionals around the world work tirelessly to respond to the COVID-19 pandemic, it is clear that traditional methods of contact tracing need to be augmented in order to help address a public health crisis of unprecedented scope. Rapidly developing digital technologies may aid public health surveillance and containment strategies for this pandemic and become part of the larger toolbox for future infectious outbreak prevention and control. The Johns Hopkins Project on Ethics and Governance of Digital Contact Tracing Technologies carried out an in-depth analysis of the technology and the issues it raises. Bringing together perspectives from bioethics, health security, public health, technology development, public policy, and law, we present clear and detailed guidelines to help manage the responsible creation, implementation, and application of digital contact tracing – prioritizing alignment of technology with public health needs and public values, building choice into design architecture, addressing equity considerations, and capturing real-world results and impacts to allow for continuous learning.

Issues of Privacy and Informed Consent in the Era of Government-Led DNA Profiling Programs
SATURDAY, 2:15 – 2:45 PM EST, ZOOM ROOM 17
FLASH PRESENTATION Q&A (LIVE) – CONSENT, PARTICIPATION, POWER, AND TRUTH TELLING
Presenter: Sheethal Jose
In 2019, the Trump administration implemented a pilot program to perform “Rapid DNA” testing on immigrants at the US-Mexico border to further justify their policy of family separation. In addition to using these tests to determine the biological relationships between migrant children and their accompanying adults, there are plans to enter the DNA information into the FBI Combined DNA Index System (CODIS) database. Similar government-led forced DNA profiling programs have been implemented in China, where the government has been collecting DNA samples from the detained Muslim Uyghur population to aid law enforcement agencies. The ethical, legal, and social implications (ELSI) of using DNA testing to profile minority populations, especially in the context of law enforcement, are significant. Major ethical concerns include privacy, informed consent, racial discrimination, and social justice issues raised due to the targeting of vulnerable populations. Forcefully subjecting an individual to genetic testing is a gross violation of privacy rights. Although migrants are provided with consent forms before being tested, they are pressured under the looming threat of separation if they do not agree to the DNA test. Obtaining consent under these context is not meaningfully voluntary. This pretense of consent should not be the standard for genetic profiling of minority populations. Rather, we need policies that capture the nuances of informed consent in the context of DNA testing and law enforcement.
Access the pre-recorded flash presentation here.
Aging and Vaccination in the US: A Roadmap for Research, Ethics, and Policy
SUNDAY, 1:00 – 1:30 PM EST – ZOOM ROOM 8
PAPER PRESENTATION Q&A (LIVE) – RESEARCH
Presenter: Alexandra Ruth
By 2030, one in five Americans will be 65 years of age or older. Older adults are highly susceptible to infectious diseases due to “immunosenescence,” the natural aging process of the immune system that increases risk for and severity of infections. Vaccination of older adults has received relatively little attention in the public health ethics literature compared to childhood vaccination, which has largely been successful through the use of school vaccine mandates and has played a key role in reducing childhood infectious disease burdens. Meanwhile, older adults in the U.S. still experience significant morbidity and mortality from vaccine-preventable diseases such as influenza, pneumonia, and shingles, yet vaccination rates in this population remain low. Factors driving low uptake of vaccines among older adults include lack of a provider recommendation; perceived unimportance of vaccination; lack of enshrined vaccine mandates similar to those that exist for childhood vaccines; and high costs of vaccines that may require cost-sharing, particularly those covered under Medicare Part D. This paper 1) articulates past and present issues in vaccine ethics, financing, and policy specifically relevant to older adults and 2) evaluates the ways in which current vaccine financing, guidelines and R&D priorities are falling short for the burgeoning aging population, using this analysis to identify specific duties and obligations that the public health community has regarding vaccination needs for an aging population. Finally, this paper synthesizes these duties and obligations into an actionable roadmap for addressing gaps in research, ethics, and policy related to aging and vaccination.
